Water Molecule
Investigate the Composition and Structure of Water
Our water molecule worksheet reviews chemistry concepts and teaches
students the structure and composition of water.
Free Download Below
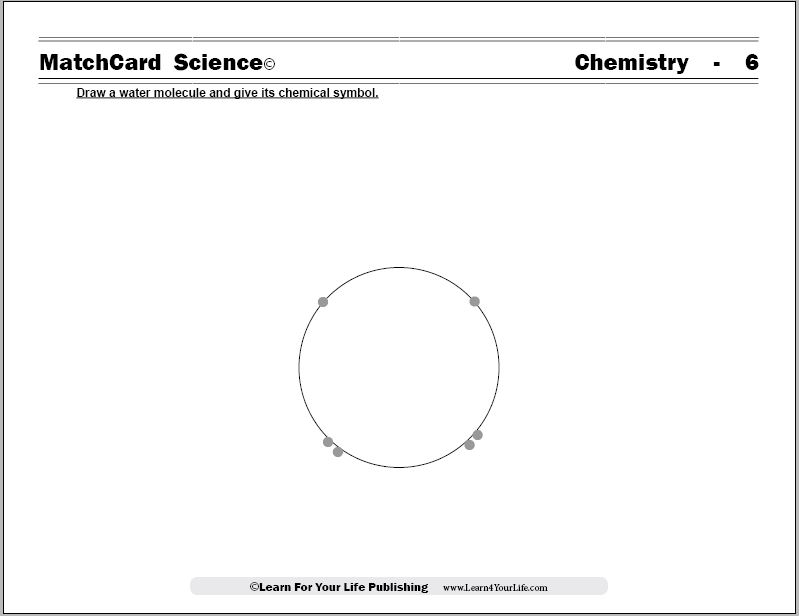

MatchCard Science Water Molecule Worksheet
Chemistry Objective #6: Draw a water molecule and give its chemical symbol.MatchCard Information Pieces are used to demonstrate the structure of the molecule and its chemical composition.
Project: Build molecule from large and small marshmallows.
Download the Water Molecule MatchCard
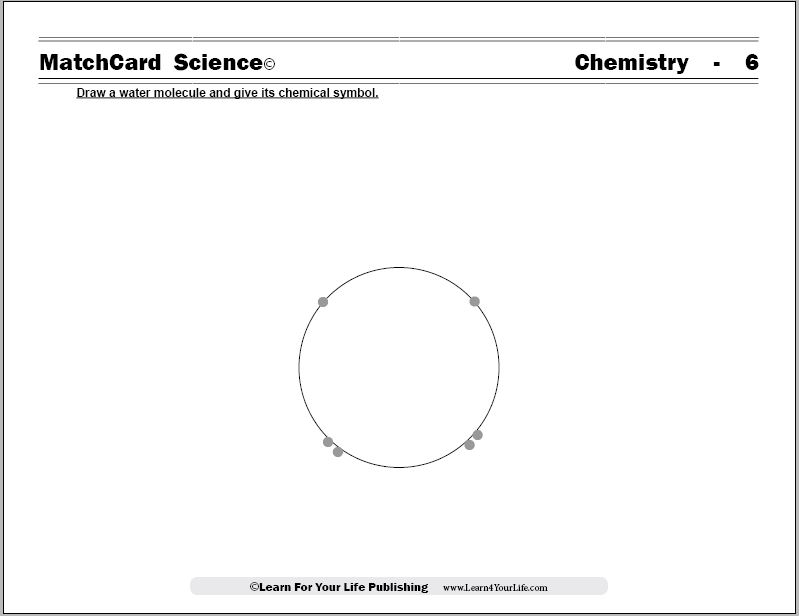

This is MatchCard #6 of the Chemistry Unit Study. Find more information on MatchCard Science below.
Name that Shape
Water Molecule Structure
To get started, draw the diagram of a water molecule on a blank sheet of paper. Or, as an alternative, you can show the diagram on the Teacher's Guide of the Water Molecule MatchCard. (Cover the chemical composition in the middle.)Ask the student what it looks like.
Most likely they will answer that it looks like Mickey Mouse ears. Explain that it is also the chemical structure of one of the most common chemicals.
If they haven't guessed it, give some other hints:
- More than 60% of your body weight is made from these molecules.
- It covers more than 2/3 of the earth's surface.
- Your famous mouse needs it to drink.
Review Chemistry Concepts
The information in this MatchCard builds on concepts learned in previous MatchCards from the Chemistry Unit Study:- Electrons are on the outside in model of an atom
- Number of electrons in a hydrogen atom
- Atomic number and symbol of an oxygen atom
- Outer shell layer and electron configuration
- Electron sharing in molecules
Now all of these chemistry concepts will come together as students build a water molecule.
Closer Look at the Water Molecules Composition
Review Oxygen
Look at the oxygen atom on the students MatchCard.RemiNd the student that it has eight electrons, two have filled the inner shell, and six are in the outershell.
Electrons are more stable when they are paired. Four of the six electrons in the outer shell are paired. The atomic symbol of oxygen is "O".
Let's Make Oxygen More Stable
Lay the two hydrogen information pieces near the MatchCard to suggest the answer to your next question.Ask the student:
Since there are two unpaired electrons in this oxygen atom, what do you think might be used to pair those electrons and make oxygen more stable?
If the student can't answer, hand him or her the hydrogen atoms. As a last resort, show the picture of the water molecule on the teacher's guide.
Quick Hydrogen Review
The student should remember that hydrogen has only one electron, and it's chemical composition is "H".Chemical Formula of Water
Students often have heard that water is "h-2-o" before they have any understanding of what that means.While looking at the structure of the molecule on the MatchCard, ask them why water is often called "H2O?"
They should be able to deduct that it is because there are two hydrogen and one oxygen atoms in this molecule.
Point out that we don't use a "1" to show there is only one of a particular atom. It is understood.
Note that subscripts are used with the number of atoms in a chemical formula.
Build Your Own H2O Molecule
Use large and small marshmallows and tooth picks to build the familiar shape. Have the students roll the marshmellows to make them rounder.Let them know that this is an important shape for more than just mouse ears. The fact that both hydrogen atoms stay causes water to have some chemical properties which are essential for life. If the three molecules lined up in a straight row, life on this planet would not exist.
Hint: Water is a polar molecule: one side has a slightly positive charge and one side has a negative charge.
MatchCard Science
How To Use MatchCards
Download the FREE MatchCard Science Instructor's Guide and see how MatchCards can make building their science knowledge base fun.
Chemistry Unit Study

Explore the building blocks of matter with the chemistry unit study.
12 Science Unit Studies

Chemistry is only one of twelve complete unit studies for kids in 3rd to 8th grade.
Comprehensive objectives, hands-on projects, suggested science fair experiments, and the fun game-like MatchCards keep them interested in learning science. See all twelve MatchCard Science Unit Studies.
About Our Site
Hands-On Learning