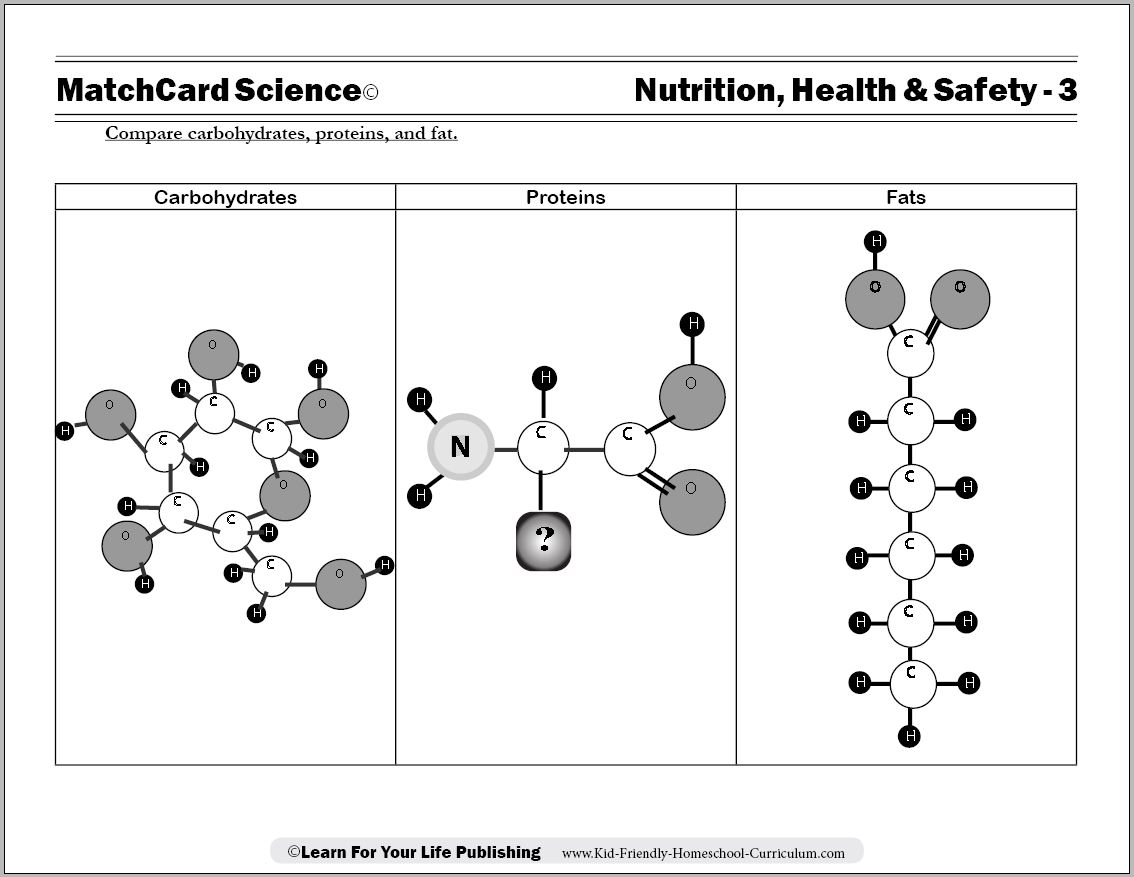
Protein, Carbohydrate, Fat Worksheet
Your middle school students will compare proteins, fats, and carbohydrates.
Free Download Below
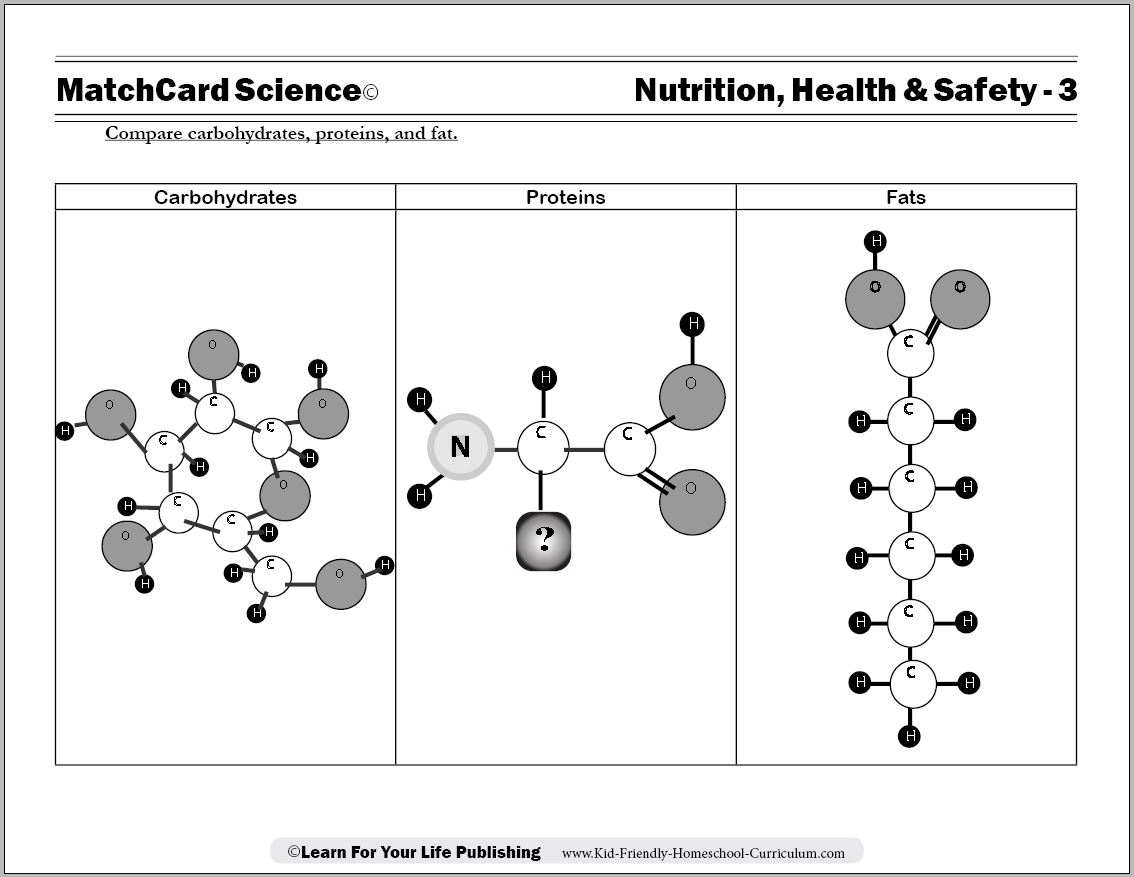

Protein Carbohydrate Fat Worksheet
Objective: Compare carbohydrates, proteins, and fat.MatchCard: Download below.
MatchCard Information Pieces describe characteristics of fats, proteins, and carbohydrates. Students match the pieces to the correct nutrient.
Print the MatchCard

Click image to go to download.
This is MatchCard #3 of the Nutrition, Health, and Safety Unit Study. Find more information on MatchCard Science below.
Carbohydrate Molecules
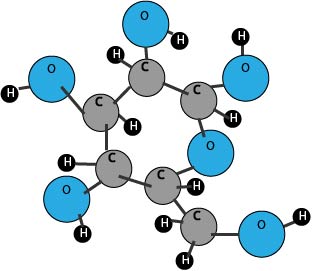
Marshmallow Carbohydrate Molecule
Carb models can be built with different colors of large and small marshmallows. Use one color of large marshmallow for carbon and a different color for oxygen. Use small marshmallows for hydrogen molecules. Toothpicks are used to join them.If you can't find commercially colored marshmallows, I have found the easiest way to dye them is to roll them in a few drops of food coloring and let them set on wax paper to dry.
An alternative is the coin method described next, which makes a fast and fun memory game. Whether you use the marshmallows or coins, keep the materials handy for another project with proteins.

You will need 6 large carbons, 6 large oxygens, and 3 large nitrogens. You will also need 12 small hydrogens.

Make the carbohydrate molecule. Take a pic and save the marshmallows for the activity below.
Memory Money Molecules
Use nickels for carbon, dimes for oxygen, and pennies for hydrogen in this exercise. Have the student(s) silently study the carbohydrate molecule for one minute. Then, without looking at the illustration, see how fast they can make it with the coins.Fill 'er Up
Where does your energy come from?
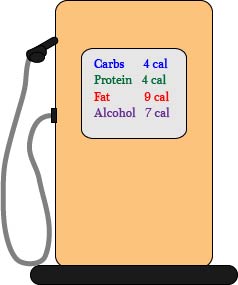
FYI Drinking alcohol has 7 calories per gram but is not included here as part of nutrition.
What is Protein?
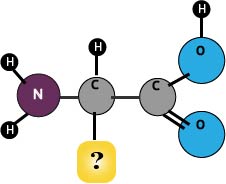
- Can be an energy source instead of carbs
- Provides the structure, or skeleton, that makes up the cells of our body
- Many hormones, enzymes, and other substances our body makes are made of proteins.
Check for Protein
You can buy Biuret Regeant Solution commercially to check for the presence of protein in different foods. You can get it on the internet or from science or chemistry supply stores for around ten dollars.
Put a variety of foods on the table, let the students guess if they would have high amounts, medium amounts or no protein. Let them check their guesses with the biuret solution.
Protein Rainbow Salad


- 15 oz can black beans
- 15 oz can chick peas or chili beans
- 15 oz corn
- 12 oz jar of mild salsa
Alphabet of Amino Acids
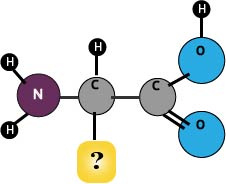
Protein Matrix


Add 2 tsp of water to gelatin, stir, then knead. A ball of protein will result.
Protein and Fat Models
If you did made the marshmallow model of carbohydrates, add a new color to stand for nitrogen and make an amino acid molecule.If you used the coins, let the nitrogen be represented by a nickle. Also make a fatty acid chain. The student should recognize that carbs, protein, and fat are made out of largely the same atoms. That is why our body can easily convert the extra calories we consume into fat.
In the same way, if a person is dieting or starving, the body will break down the fat stores, convert them into glucose, and the cell can use that for energy.

Make 3 amino acids with the materials from your carbohydrate molecule and the 3 nitrogen molecules.
What Are Fatty Acids?
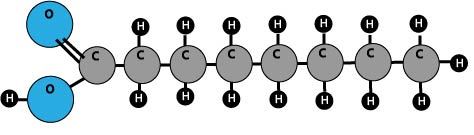
Fatty acids are made of carbon chains of differing lengths. They are considered saturated if all of the carbon atoms are saturated with hydrogen atoms. Unsaturated fats do not have all of their carbon atoms saturated by bonding to hydrogen atoms. Unsaturated fats are healthier for your heart.

Make a fatty acid chain.
Butter. Margarine. Butter.



- Melt half a cup of margarine and half a cup of butter and keep them in separate containers.
- Do a blind taste test and see if you can identify which is which. (Generally we do not taste science experiments, but you know these are food products.)
- Add a drop of both to a napkin or sheet of paper. Observe what happens.
- Mix one tablespoon of each with one tablespoon of water. What happens.
- Mix one tablespoon of butter and one tablespoon of margarine. Which mixes with which?
- Leave both out for several hours. Which will resolidify?
Fat is Flavor
The properties of fat help to give food flavor. Try eating cooked noodles with and without butter or margarine. Which tastes better?Sprinkle both with parmesan cheese. Do you taste the cheese better with or without the butter?
Keep in mind how many extra calories are in fat. Think of how much fat you want to add to your diet in order to temporarily please your taste buds.
Hydrophobic Fat: Avoids Water
Activity: Try Mixing Fats and Water
Water and fats do not mix well. Try it. In a tall, thin container add some cooking oil. Add water that has been dyed blue or any other color. Watch how they separate from each other.Notice how many salad dressings have their oil and water mixtures separated and need to be mixed by shaking. They won’t stay together too long.
Why? Water molecules are polar with slight charge - almost like a magnet - one side is positive and one is negative. Substances that mix easily with water usually have a charge as well. Fats do not have a charge, hence will not mix.
Fun With Food Dye
Here’s a fun experiment. Pour milk into a wide container like a pie dish. Gently add drops of different food coloring to the milk. Try not to disturb the surface. Now, add a single drop of dish soap to the milk. The colors will begin to swirl as the hydrophobic (water hating/fat loving) end of the soap molecules bind to the fat molecules in milk. When the reaction ends, add another drop to the dish again and the reaction continues.Try with different types of milk: whole milk, cream, fat free milk. You can write a hypothesis about how the amount of fat in the different types of milk will affect the food dye and create a science experiment.
Soap, Water, and Fat
Soap is a unique substance. One part of its molecules have a charge, so it is considered hydrophilic (water loving.) The other part of a soap molecule does not have a charge and is water hating (hydrophobic) and fat loving.Do you see how dish soap cleans your plate after a greasy meal? A molecule of soap attaches to a molecule of fat. The other end of the soap molecule attaches to the water and sweeps the grease away. Try washing a greasy plate with and without dishsoap.
MatchCard Science
How To Use MatchCards
Download the FREE MatchCard Science Instructor's Guide and see how MatchCards can make building their science knowledge base fun.
Nutrition, Health, and Safety Unit Study

See more about the Nutrition, Health, and Safety Unit Study.
12 Science Unit Studies

Chemistry is only one of twelve complete unit studies for kids in 3rd to 8th grade.
Comprehensive objectives, hands-on projects, suggested science fair experiments, and the fun game-like MatchCards keep them interested in learning science. See all twelve MatchCard Science Unit Studies.
About Our Site
Hands-On Learning