Physical and Chemical Changes
Download the Chemical And Physical Changes MatchCard
With the Physical and Chemical Changes worksheets students differentiate between a chemical change and a physical change.
Free Download Below
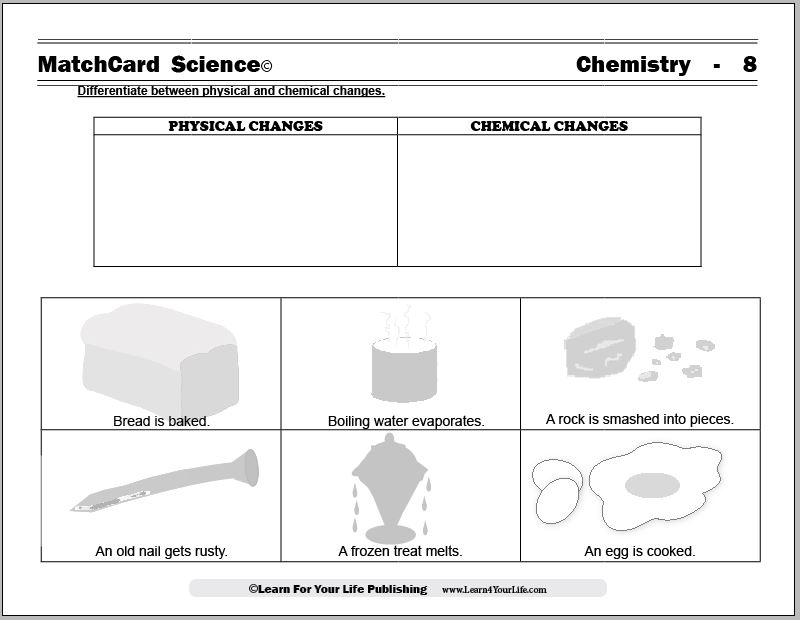

MatchCard Science Physical and Chemical Changes
Objective of this lesson: Differentiate between physical and chemical changes.The MatchCard lists different changes which students identify as a chemical change or a physical change. Students will identify each example as physical or chemical.
Projects: Fry an egg, make some toast, rust a nail. Go on a physical or physical change hunt and make a poster.
Download and Use the Physical and Chemical Changes MatchCard
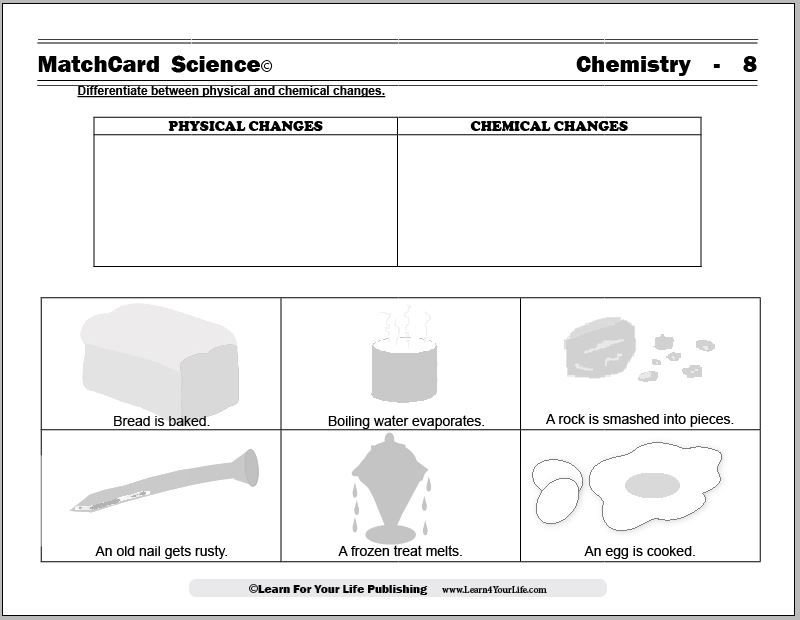

This is MatchCard #8 of the Chemistry Unit Study. Find more information on MatchCard Science below.
Introductory Activity
Fry An Egg
You can introduce the concept of physical and chemical changes with this simple kitchen activity: frying an egg.Start by having the student melt butter in a frying pan. Ask them what happened to the butter.
Answer: The heat caused the molecular bonds to pull further apart. The melted butter has the same molecules, but the bonds between them are not as close. Concepts of melting, evaporation, condensation, and freezing can be quickly reviewed.
An important point to emphasize is that the melted butter is made up of the same chemicals it was made up for before. Just like water is still H2O in the solid, liquid, or gaseous state; the butter is still the same. Now carefully crack open an egg, slide it into the melted butter, and watch it fry. This is particularly more effective if the egg whites and yolk can be seen distinctly.
As the egg white is cooking and changing, ask what is happening.
Answer: The substance of the egg is changing. The atoms are leaving the molecules because of the chemical changes caused by the heat. The atoms form new molecules with other atoms. The cooked egg white is a different substance, made out of a different type of atoms.
We will learn more about molecular changes later in the chemistry unit study.
Make Some Toast
We can further illustrate the difference between physical and chemical changes as we make some toast to go with the fried egg.Have them put two pieces of toast in a toaster and toast it to the desired level of crispness. Right before it comes out, take another piece of untoasted bread and tear it into two pieces.
As the toast comes out, ask them which piece of bread has undergone a physical change and which piece of toast has undergone a chemical change.
It is easy for the students to detect that the broken bread is merely a physical change while the toasted bread has had a chemical change on its surface.
Discuss the difference between the two types of changes while enjoying your fried egg sandwhich (or whatever physical changes you wish to make to serve your snack.)
Another Chemical Change: Rust a Nail
Here is another interesting chemical change that will take a little longer:Put a small nail in a glass of water. Use a disposable cup or glass jar.
The nail will rust with time, which is a chemical change. .
Detective Hunt
After learning the definitions and common examples of chemical and physical changes, the students can go on a detective hunt to see if they can identify other examples.
Where can you find other changes? Direct their attention to the:
- Kitchen
- Garden
- Garage
- Bathroom and household cleaners
They can make a list or poster and continue to add examples as they find them through the completion of the unit study. (Or all year. On-going review and expansion of knowledge is part of MatchCard Science.)
MatchCard Science
How To Use MatchCards
Download the FREE MatchCard Science Instructor's Guide and see how MatchCards can make building their science knowledge base fun.
Chemistry Unit Study

Explore the building blocks of matter with the chemistry unit study.
12 Science Unit Studies

Chemistry is only one of twelve complete unit studies for kids in 3rd to 8th grade.
Comprehensive objectives, hands-on projects, suggested science fair experiments, and the fun game-like MatchCards keep them interested in learning science. See all twelve MatchCard Science Unit Studies.
About Our Site
Hands-On Learning