Periodic Chart
Use the Periodic Chart In the MatchCard Science Chemistry Unit Study to study the atoms
Free Download Below
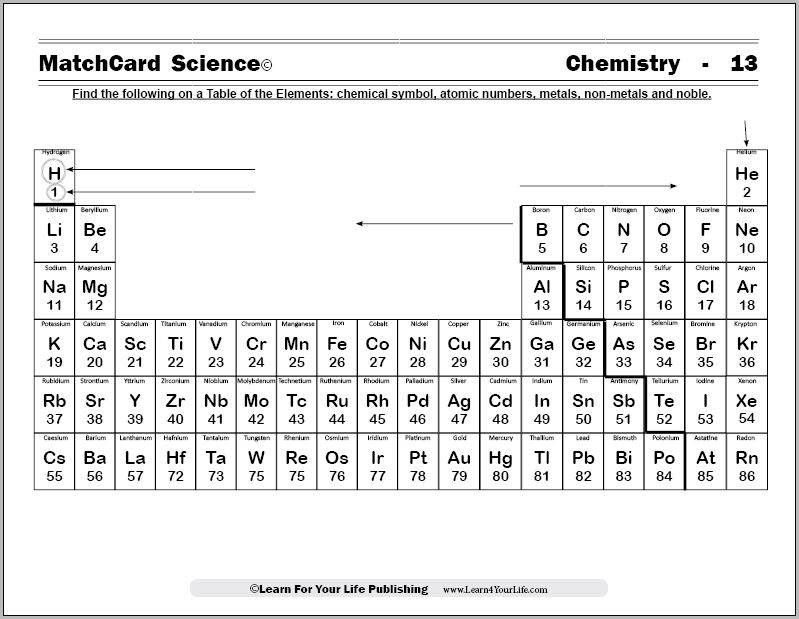

MatchCard Science Periodic Chart
Objective: Find the following on a Table of the Elements: chemical symbol, atomic numbers, metals and non-metals.MatchCard: Download below.
MatchCard Information Pieces are placed on the periodic chart as students become familiar with the content.
Project: Memory game of common elements.
Download and Use the Periodic Table MatchCard


This is MatchCard #13 of the Chemistry Unit Study. Find more information on MatchCard Science below.
What Is On The Periodic Chart?
Print your copy of the MatchCard Science Periodic Table (below) and show it to your students. Do they recognize it?Explain that the Periodic Chart organizes much of the information they have already learned in their chemistry unit study. They can find:
- Chemical Symbols
- Atomic Number
- Metals grouped together
- Non-metals grouped together
What Is A Metal?
Ask your student what a metal is. It is something we all seem to know, but might have a hard time describing.By definition a metal conducts electricity, which seems a bit unrelated to the periodic table. But if you stop and think of what we have learned in previously Chemistry MatchCards, it makes sense.
A metal conducts electricity.
Metals lose electrons to make a positive ion.
You can see that the metals are on the left side of the chart. They have the extra electrons in the outer shells When they "give" those electrons to another molecule, they have more protons in the nucleus than electrons circulating in the electron shells.
Have the students shade the metals yellow with a colored pencil.
So What's A Non-metal, then?
Okay, the metals stick together on the left side of the periodic table, leaving the non-metals to hang out on the right side.Those on the right side of the chart ALMOST have their outer shell filled with electrons. They get mighty greedy for another electron so they can fill up their shells. That's why they take the electrons away from the metals over on the left side of the chart.
The result: Well, now they have more electons than they have protons, leaving their ions with a negative charge.
Have the students shade the non-metals with a blue colored pencil.
Transitional Metals
The elements near the line separating metals and non-metals are generally referred to as transitional metals. They may be shaded green as a combination of the metal and non-metal colors. They include: boron, silicon, germanium, arsenic, antimony, and tellerium.Nobel Gases
There are six nobel gases on the far right of the periodic table. What is so special about these non-metals?Their electron shells are completely full. They are said to be stable. They generally neither give or take electrons. Shade the nobel gases with red. Since all the non-metals were previously shaded blue, it will give these special elements a purple shade.
Name that Element
Previously students learned the names and atomic number of certain elements (hydrogen, helium, sodium, carbon etc.)Here is a game that will expand their knowledge of chemical names and symbols. Take index cards, and write the names of the following chemicals on one card and the symbol on another card.
- Mg - Magnesium
- Cl - Chlorine
- Al - Aluminum
- K - Potassium
- Mn - Manganese
- Cu - Copper
- Au - Gold
- Fe - Iron
- I - Iodine
- Ag - Silver
- Sn - Tin
- Pb - Lead
MatchCard Science
How To Use MatchCards
Download the FREE MatchCard Science Instructor's Guide and see how MatchCards can make building their science knowledge base fun.
Chemistry Unit Study

Explore the building blocks of matter with the chemistry unit study.
12 Science Unit Studies

Chemistry is only one of twelve complete unit studies for kids in 3rd to 8th grade.
Comprehensive objectives, hands-on projects, suggested science fair experiments, and the fun game-like MatchCards keep them interested in learning science. See all twelve MatchCard Science Unit Studies.
About Our Site
Hands-On Learning