Molecules and Compounds
Compare Elements, Compounds, and Mixtures
Chemistry MatchCard 7 explains the relationship between molecules and compounds.
Free Download Below
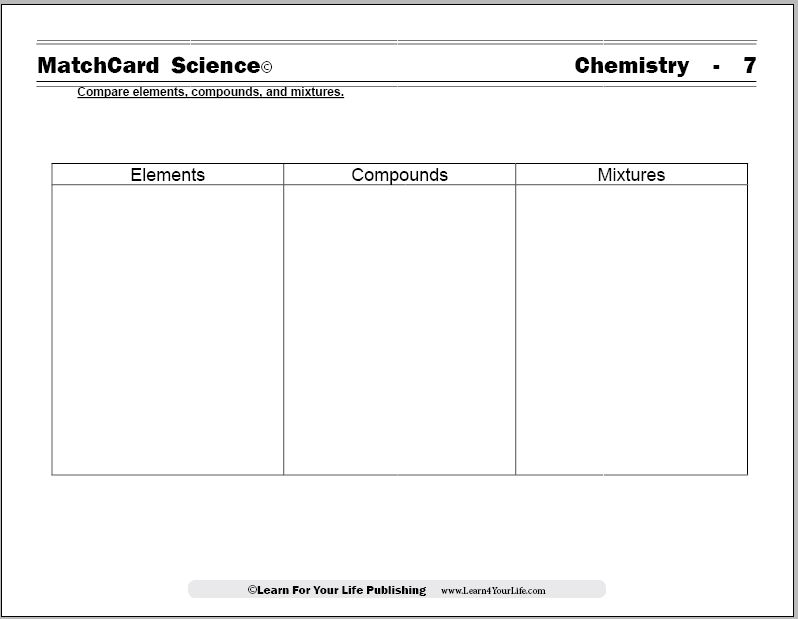

Molecules and Compounds MatchCard
Objective: Compare elements, compounds, and mixtures.MatchCard: Download below.
MatchCard Information Pieces define elements, compounds, and mixtures and give common examples of each. Students match definitions and examples with the correct term.
Projects: Mix and unmix a mixture. Go on a detective hunt for compounds and mixtures.
Download and Use the Elements Compound and Mixtures MatchCard


This is MatchCard #7 of the Chemistry Unit Study. Find more information on MatchCard Science below.
Let's Make a Mixture
Let's start our chemistry lesson by making a nice cold glass of lemonade. Sure, you can squeeze the lemons together and add the sugar and water. For our purposes, however, a powdered lemonade mix will work just as well.Have the students slowly stir the ingredients and watch them interact as they mix.
As they enjoy their beverage, you can tell them they are consuming a mixture. Ask them why it is called a mixture. Answer: Two substances were mixed.
Point out that both the water and lemon substance are still detected in the new mixture they have formed.
Make another mixture: salt water. Have them combine the salt and water. Can they taste the salt?
Again note that the salt and the water both maintain their physical properties when the mixture is formed.
Put the salt water aside. You are going to "unmix" it later.
Now, explain that scientists use compounds to make mixtures.
Molecule Vs. Compounds
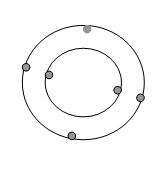
Elements
A element is a substance whose atoms are all alike. Each atom has the same number of protons and electrons.The elements are all listed on the periodic table according to the number of protons and electrons. The only elements known to humanity are on the periodic table.
Common examples of elements that your students are familiar with include: gold, silver, aluminum, iron etc.
Compound
Students learn the definition of molecules in the Molecule MatchCard.A molecule is two or more atoms that share electrons. The definition is important to understand the difference between molecules and compounds.
A compound is a substance made out of molecules.
Hmm, did you get that? Sounds like an obvious statement but for kids trying to understand the difference between compounds and mixtures, it can get confusing. Another way of looking at it is that a compound is a substance which is made up entirely of the same type of molecules.
Some of the most common substances are compounds: water, salt, minerals. Most of the chemicals you can buy are compounds. All particles in the compound are the same: they are made of the same molecules. Compounds do not have the same characteristics as the elements from the atoms the molecules are made. For example, water is made out of hydrogen and oxygen. But you cannot detect either hydrogen or oxygen in a glass of water.
Salt is made of sodium and chloride. Neither of those two elements are detected in a container of salt.
Mixtures
Our life is filled with mixtures. It is a substance made out of more than one compound.Mixtures are often man-made chemicals. The ingredients in the substance continue to display their own characteristics.
For instance, the salt and the water are both detected in salt water.
Mix and Unmix
Mixtures are made of ingredients that can be mixed, and often then can be unmixed.Take the salt water. Ask the students if they can think of a way to unmix the two.
A simple way to do it is to boil the mixture until the water evaporates. The water will go into the air leaving the salt behind in the pan.
Let's Hunt for Elements, Compounds and Mixtures
Go on a detective hunt through your bathroom, kitchen, and garage. See if students can make a list of elements, compounds and mixtures.Hint: You may want to review the elements on the periodic table beforehand if you are not familiar with all of them.
MatchCard Science
How To Use MatchCards
Download the FREE MatchCard Science Instructor's Guide and see how MatchCards can make building their science knowledge base fun.
Chemistry Unit Study

Explore the building blocks of matter with the chemistry unit study.
12 Science Unit Studies

Chemistry is only one of twelve complete unit studies for kids in 3rd to 8th grade.
Comprehensive objectives, hands-on projects, suggested science fair experiments, and the fun game-like MatchCards keep them interested in learning science. See all twelve MatchCard Science Unit Studies.
About Our Site
Hands-On Learning