Molecule Worksheets
Compare molecules and atoms with this download.
Students identify the characteristics of molecules on these MatchCard Science molecule worksheets.
Free Download Below
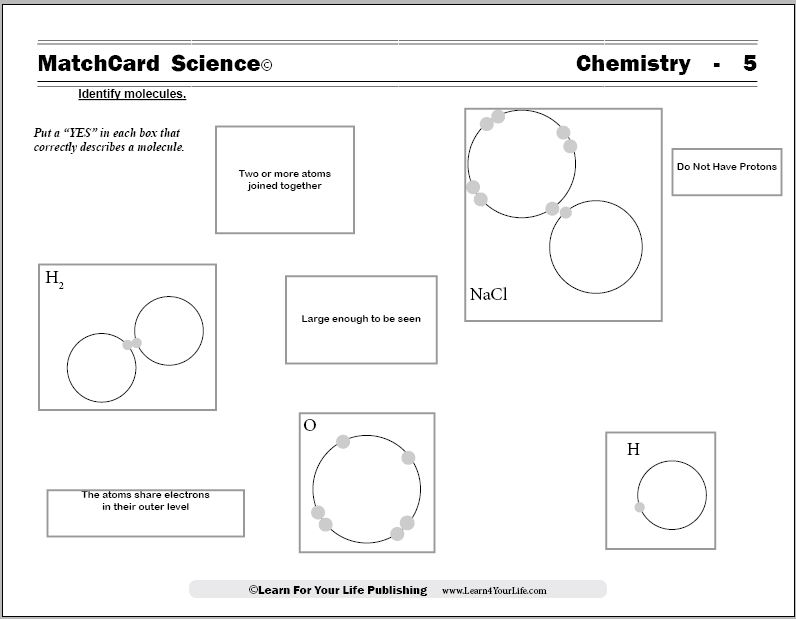

MatchCard Science Molecule Worksheets
Objective: Identify molecules.MatchCard: Download below.
MatchCard Information Pieces describe the characteristics of molecules. Students match "Yes" or "No" cards to the statements on diagrams on the worksheet.
Projects: Build a paper plate model of sodium chloride to demonstrate how molecules share electrons in the outer shell.
Print the Molecule Worksheets From MatchCard Science


This is MatchCard #5 of the Chemistry Unit Study. Find more information on MatchCard Science below.
What's a Molecule?
To understand this concept, students need to know concepts presented in previous lessons of this Chemistry Unit Study: When a molecule is formed, two or more atoms share an electron in the outer shell. Often (but not always) this causes both atoms to have a completely filled outer shell.
Let's Make a Molecule
Before looking at the molecule worksheets, have students look at the chlorine atom in the Electron Configuration Chemistry Worksheet.
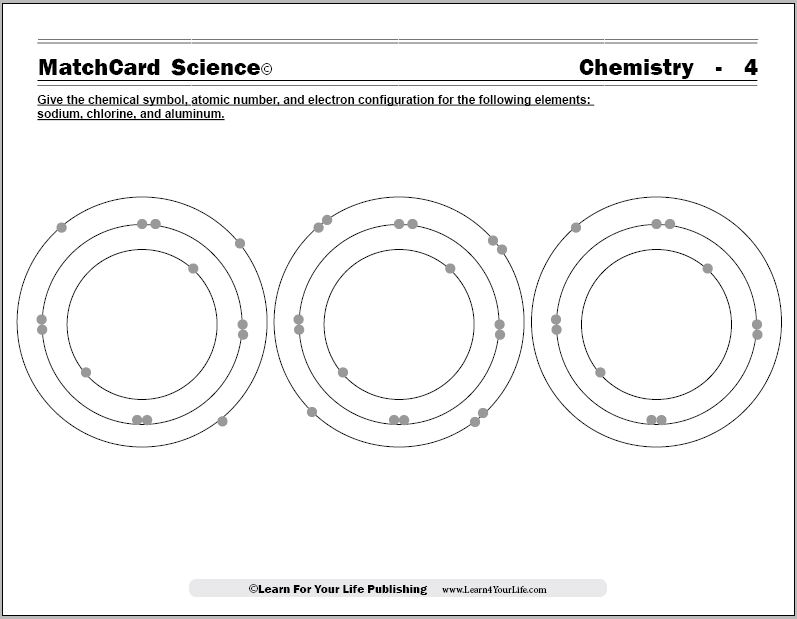
Discuss that atoms are more stable when the electron shells are completely filled.
Then look at the sodium atom which is also depicted in the electron configuration worksheet. What does it have in its outer shell? (Answer: one electron.)
Explain that if a sodium atom would "share" it's outer electron with a chlorine atom, a molecule would be formed. That molecule, sodium chloride, is much more stable than either sodium or chloride atoms because the outer shell is filled.
Draw A Sodium Chloride Molecule
On a piece of paper, draw a complete molecule of sodium and of chloride using the same type of circles used for our paper plate models.Have the models line up so the sodium electron in the outer shell is paired with the unpaired electron in the chlorine atom.
Show them the picture of sodium chloride in the middle of the molecule worksheets.
A Simpler Molecule Model
Point out that the diagram in the molecule worksheets does not have all of the electrons.Models often have only the electons in the outer shells represented. This is because all of the inner shells are filled. It is understood that those elecrons are present and make up the correct atomic number.
However, we can make a faster and simpler model if we show only the outer layer.
More About Molecules
Remind students that electrons do not stay in one place as they do in our model. They circulate around the nucleus at an extremely fast speed.Shared electrons will circulate around both atoms. This creates a strong bond which is what causes the atoms to stay together.
Pass the Sodium Chloride
Ask if they have ever heard of sodium chloride.It is, of course, simple table salt. The chemical name is sodium chloride. It has a chemical symbol NaCl.
Sodium chloride is a very important chemical. In addition to being in the salt shaker, it is in every drop of ocean water, and in every cell of the human body.
Get a crystal of salt. Ask if they think this is a molecule. Younger students might at first think they are holding a molecule in their hand. Explain that there are millions of molecules in that tiny salt crystal.
You might want to do a chemistry hunt and see how many food or cleansers in your home have sodium chloride on the label.
Hydrogen Molecules
Molecules can be made of atoms from the same or different elements.Hydrogen molecules are two hydrogen atoms that have joined to share electrons.
Look at the diagram of the hydrogen molecule in the molecule worksheets. Note that each contributes one electron; and that the inner most electron shell is filled with only two electrons.
Hydrogen gas is very abundant on this planet. It exists as molecules of two hydrogen atoms bonded to fill their electron shell. It is written as H2 to show the number of atoms in each molecule.
Use the Molecule Worksheets
Before using the molecule worksheets as a written assignment, make it a game.Place four sheets of paper on the floor, spread out. Each sheet should have one of these words on it:
- Molecule
- Atom
- Both
- Neither
Here are the descriptions from the molecule worksheets:
- Two or more atoms joined together (Molecule)
- Large enough to be seen (Neither)
- Diagram of two hydrogens (Molecule)
- Diagram of one oxygen (Atom)
- Sodium Chloride (Molecule)
- Do not have protons (Neither)
- The atoms share electrons in their outer level (Molecules)
- Have a nucleus (Both)
- Single helium (Atom)
- Protons are in the nucleus (Both)
Coming Up
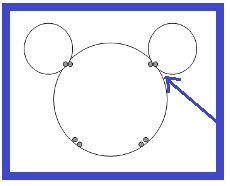
The next lesson will introduce students to a very important molecule with the famous mouse ear shape: water molecules.MatchCard Science
How To Use MatchCards
Download the FREE MatchCard Science Instructor's Guide and see how MatchCards can make building their science knowledge base fun.
Chemistry Unit Study

Explore the building blocks of matter with the chemistry unit study.
12 Science Unit Studies

Chemistry is only one of twelve complete unit studies for kids in 3rd to 8th grade.
Comprehensive objectives, hands-on projects, suggested science fair experiments, and the fun game-like MatchCards keep them interested in learning science. See all twelve MatchCard Science Unit Studies.
About Our Site
Hands-On Learning