Model of Atom
Electrons, Protons, and Neutrons Worksheet
This model of the atom shows the electrons, protons and neutrons. Worksheet and activities are from the MatchCard Science Chemistry Unit Study and teach the parts of the atom for students in 3rd to 8th grade.
Free Download Below
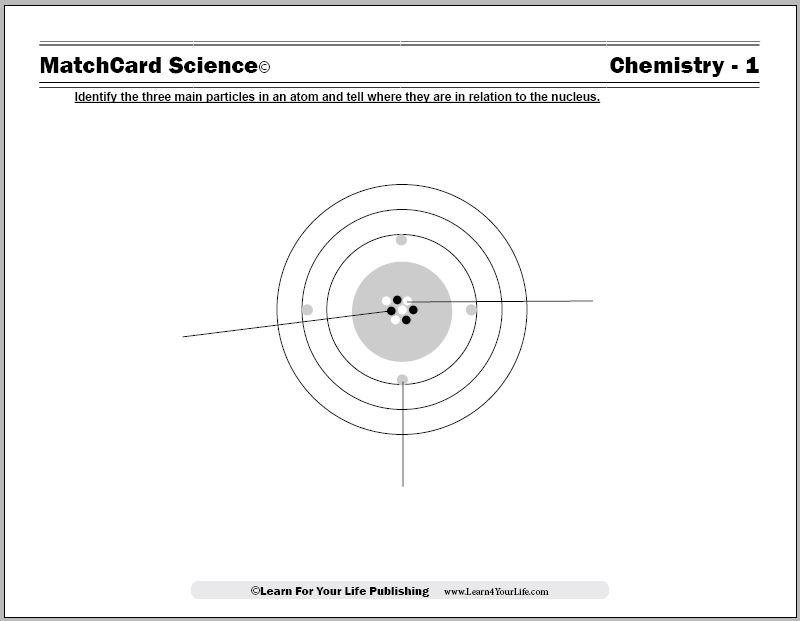

The Model of the Atom Worksheet
Objective: Identify the three particles in an atom and tell where they are in relation to the nucleus.MatchCard: Download below.
MatchCard Information Pieces identify and describe protons, electrons, and neutrons.
Projects: Make a paper plate model of an atom and play a dice game building atoms.
Download and Use the Model of Atom MatchCard


This is MatchCard #1 of the Chemistry Unit Study. Find more information on MatchCard Science below.
Parts of the Atom
Teaching the Model of the Atom
This lesson introduces students to the three main parts of the atom: the electrons, protons, and neutrons. Students learn that atoms are the basic building blocks of all matter.We can compare an atom to the solar sytem, which is easier for children to visualize. The sun is the nucleus, or the center, of the atom. Electrons circulate around the nucleus somewhat like planets circulate around the sun.
Electrons
Electrons circle around the nucleus.They circulate in electron levels. The levels are like rings or orbits around the nucleus.
In most atoms, there are the same number of electrons as protons. In ions, the number of electrons has increased or decreased giving the ion a negative or positive charge.
Electrons have a negative charge.
Protons
Protons are in the nucleus of the atom.There are the same number of protons as there are electrons. As mentioned above, an ion has gained or lost electrons so the number will not be the same. This is a more advanced concept than we are teaching in this first lesson.
Protons have a positive charge.
Neutrons
Neutrons are in the nucleus.The number of neutrons in an atom may change. (This is called an isotope of the atom. It is a more advanced concept than is taught in elementary chemistry.)
Neutrons have no charge. They are neutral.
Let's Make a Model
Building model of atoms from paper plates
This model of an atom will be made with a paper plate.
For the protons, neutrons, and electrons, we will use three different colors of paper. Construction paper or any other colored paper will work fine. Using a round hole punch to punch out circles to represent parts of the atom works fine.
Write "P", "E", and "N" on the circles of paper to represent protons, electrons, and neutrons.
As a simple alternative, you can use nickels, pennies, and dimes in this atomic model. Students need to realize that the different physical sizes or monetary value are not relevent to this model.
Cut out a small black or gray circle and glue it to the center of the paper plate. It should be about 1/6 the size of the plate. The dark circle represents the nucleus.
Draw three concentric circles around the nucleus. These are the electron levels. They are like the "paths" that the electrons use to circle around the nucleus.
Activity with our Atomic Model
Here's a simple activity to introduce them to the atom.
Role a dice. Whatever number they get is the number of protons that they will have in the nucleus at the end of this activity. It is their goal.
Put the circles representing protons, neutrons, and electrons in a bag or bowl. One at a time they reach in without looking and pull out ONE circle.
They cannot have more than one proton more than electrons; or more than one electron than proton. For instance, if they have three protons and two electrons and they pull out another proton, they need to put it back.
They can always add a neutron to their model, regardless of the number of parts already on the paper plate.
When they obtain the correct number of protons and electrons as their target number, they are done.
For fun, this can be played as a game between two different players.
Compare our Atomic Model with a Real Atom
There are several differences between our model and a real atom. Ask if the students can explain the differences before telling them what they are.
Size
Obviously, our paper plate is a lot bigger than an atom. Several million atoms are in a grain of salt. Let them hold a grain of salt and imagine the size difference.
Scale
Not only is our model of atom extremely large, the parts are not to scale. It has been estimated that if an atom was as large as a football stadium, the nucleus would be the size of a marble in the center of the field, and the electrons would be the size of gnats flying around the stadium.
Three Dimensional
Real atoms are three dimensional whereas our model is flat and 2 dimensional. It is similar to the difference between a globe and a map.
Shape of the Electron Levels
While we have portrayed the electron levels as flat circles all going in the same direction, electron levels are not parallel to each other. In this way they differ from planets as well. The different levels are on totally different planes.
MatchCard Science
How To Use MatchCards
Download the FREE MatchCard Science Instructor's Guide and see how MatchCards can make building their science knowledge base fun.
Chemistry Unit Study

Explore the building blocks of matter with the chemistry unit study.
12 Science Unit Studies

Chemistry is only one of twelve complete unit studies for kids in 3rd to 8th grade.
Comprehensive objectives, hands-on projects, suggested science fair experiments, and the fun game-like MatchCards keep them interested in learning science. See all twelve MatchCard Science Unit Studies.
About Our Site
Hands-On Learning