Fusion and Fission
The Fusion and Fission MatchCard introduces the concept of
nuclear energy for students in 5th - 8th grade.
Free Download Below

Nuclear Energy
Objective: Contrast fusion and fission.The goal of this MatchCard is to introduce nuclear energy as one of the types of energy used. Further study of this topic will be done at the high school level.
Note: This MatchCard is for students in 5th grade and over who have previously had some exposure to elementary chemistry and realize an atom has protons in the nucleus and electrons circulating outside.
Background: Chemical bonds occur when two atoms share electrons circulating OUTSIDE of the nucleus. Chemical bonds require energy to form and relesae energy when broken. Nuclear energy occurs because of the fusion or fission of the protons INSIDE the nucleus. This requires or produces millions times more energy and does not occur under usual conditions on this planet.
Download the Fusion and Fission:
Nuclear Energy MatchCard
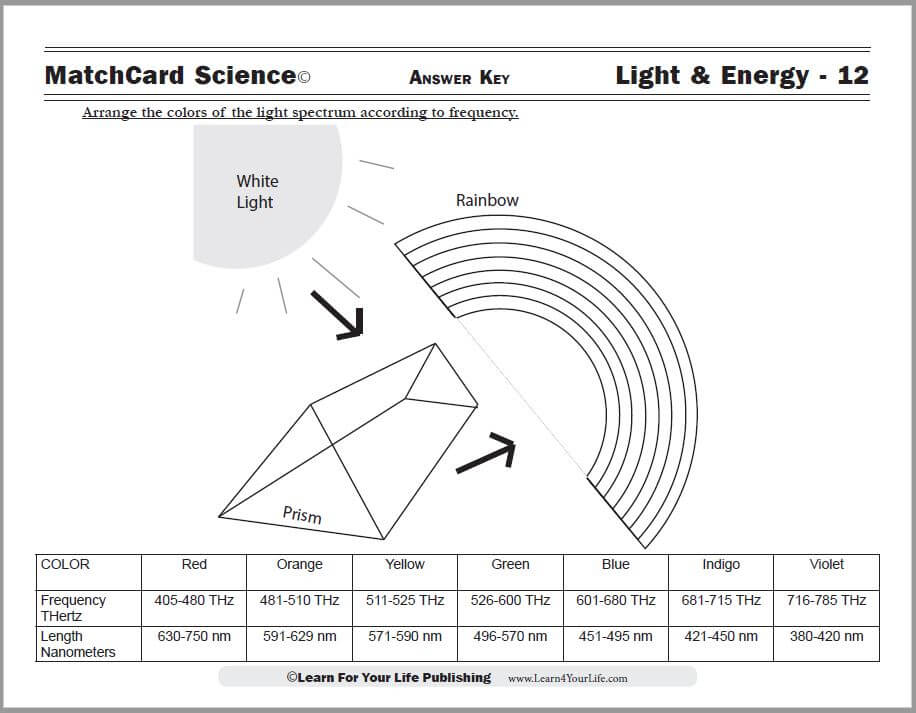

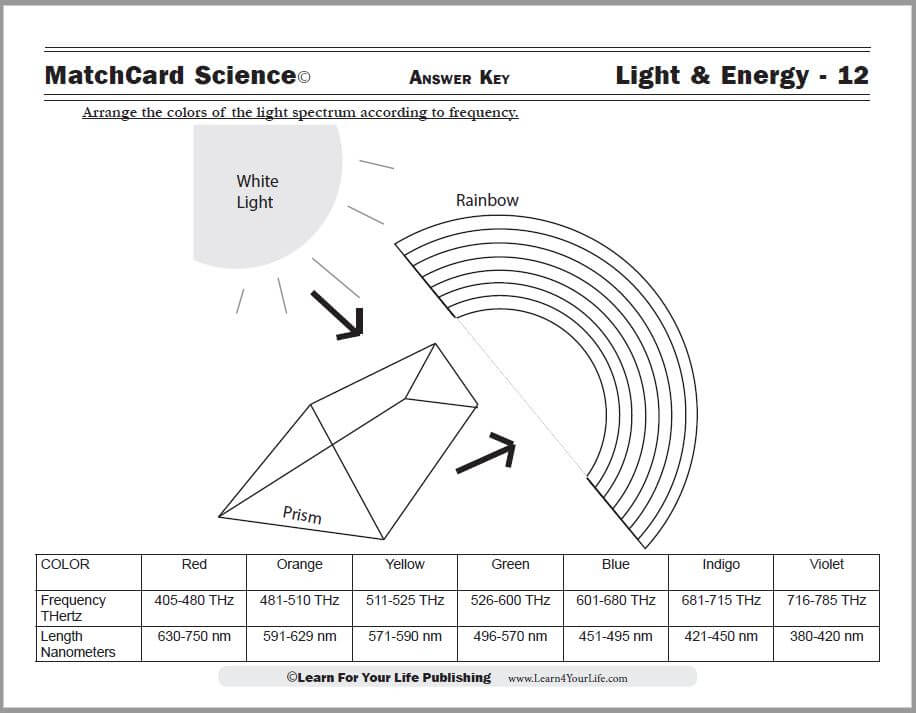
This is MatchCard #12 of the Energy Unit Study. Find more information on MatchCard Science below.
Fusion vs Fission
Chemistry Review
This lesson is for older students who have who elementary chemistry. Here are some basic facts about chemistry that you should review before studying nucleaur energy.- There are a limited number of atoms. All atoms of the same type have the same number of protons in them.
- Atoms can combine to form molecules.
- Molecules change in chemical reactions. While the molecules change and bind with different atoms, the atoms themselves do not change.
Fusion
Fusion is the union of two nuclei and requires a tremendous amount of energy to occur. This only occurs on the sun or stars where the intense heat causes two atoms to fuse. In this way, two hydrogen atoms which both have one electron and one proton are fused to produced a helium atom with two protons and two electrons. With greater heat expenditure, larger atoms will be produced.Helpful Hint: Fusion sounds somewhat like "union" and can be remembered because two nucelei are united.
Fission
Fission is the division of the protons in a nucleus to produce two smaller atoms. The splitting of the atom produces tremendous amounts of energy. Nuclear power plants usually split uranium, the atom which has 235 protons in the nucleus.Why is uranium used? It is easier to split uranium, because the large amount of protons in the nucleus are less stable. Helium, which has only two protons in the nucleus, is very stable. At this point, there are no conditions on this planet that would enable helium to be split.
Helpful hint: Fission rhymes with division, and is the dividing of the atom.
Energy Lost or Gained?
It requires energy to combine the atomic nuclei. That is why it only occurs on a star where there is a tremendous amount of heat.When an atom is split, that releases energy. Nuclear power plants split atoms to produce electricity for a city. Nuclear weapons split atoms to produce powerful bombs.
It is similar to chemical bonds that produce or require energy. When a molecule is formed it requires energy to create the chemical bond. When the molecule is split energy is released. Remember though, that a chemical reaction occur as atoms form different molecules but the nuclei of the atom is not changed. In a nuclear reaction that actual proton of the atom is either split or combined.
Hands On Activities
While MatchCard Science promotes hands-on learning for science exploration, there is an obvious limit to the ability to conduct experiments with nuclear energy. Here are some other ideas for activities to engage your student.Uses of Nuclear Energy
- There are more than 65 nuclear power plants in the world.
- They are estimated to produce between ten to fifteen percent of the world’s energy.
- What nuclear power plant is closest to you?
- Do they have a visitor center, website, or Youtube video you can visit?
Safety of Nuclear Energy
Nuclear energy is considered a clean and inexpensive form of energy. While often considered safe, there were three nuclear accidents that have left people feeling uncertain about the safety and reliabilty of nuclear power plants. You may want to look up at least one of these accidents:- Three Mile Island (Pa, USA) 1979
- Chernoybl (Russia) 1986
- Fukushima (Japan) 2011
Poster of Nuclear Fusion
Uranium is an atom with 235 atoms. It can be divided into two smallers atoms with 135 and 100 protons.Your student may wish to build a model or poster of the division of a uranium atom.
Nuclear Weapons
Nuclear weapons are called weapons of mass destruction because of the amount of damage they can do. They have only been detonated twice in war, both times in 1945 by the United States to end World War II. Their use as a weapon remains controversial.The detonation of a nuclear weapons begins with the thermal flash of the characteristic mushroom cloud that emits heat, light, and electromagnetic radiation. It is followed by shock waves that spread out in a 360 degree angle. Wide-spread fires of combustible materials follow, which in addition to causing further damage also appear to cause tornado like heat waves as air is needed to fuel the fires.
Students who are interested, may want to read about the experiences and testimony of survivors of Hiroshima and Nagasaki, Japan; where the two nuclear bombs were set in August 1945.
Fusion & The Period Table
Show the students the periodic table. The lightest element is hydrogen. On the sun, two hydrogen atoms are fused to create helium. At the core of hot stars, the heat and pressure allow helium and other atoms to fuse to form heavier elements.MatchCard Science
How To Use MatchCards
Download the FREE MatchCard Science Instructor's Guide and see how MatchCards can make building their science knowledge base fun.
Light and Energy Unit Study

Download the entire Light and Energy unit study.
12 Science Unit Studies

Chemistry is only one of twelve complete unit studies for kids in 3rd to 8th grade.
Comprehensive objectives, hands-on projects, suggested science fair experiments, and the fun game-like MatchCards keep them interested in learning science. See all twelve MatchCard Science Unit Studies.
About Our Site
Hands-On Learning