Evaporation and Condensation
Evaporation, Condensation, Freezing and Melting Science MatchCard
Explore the states of matter with the Evaporation and Condensation Matchcard.
Free Download Below

Freezing, Melting, Evaporation
and Condensation MatchCard
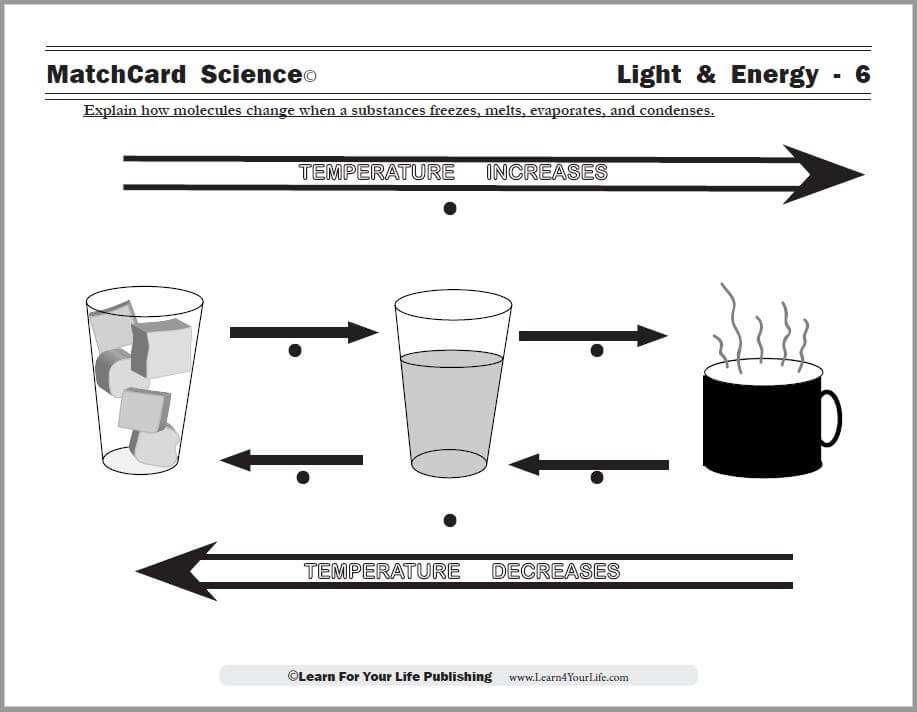
Objective: Explain how molecules change when a substance freezes, melts, evaporates, and condenses.This cool science experiment will demonstrate some of the changes a substances undergoes as it changes from solid to liquid to gas.
Print the Evaporation, Condensation,
Freezing and Melting MatchCard


Click image to go to download.
This is MatchCard #6 of the Light and Energy Unit Study. Find more information on MatchCard Science below.
Experiment with the States of Matter
Freezing
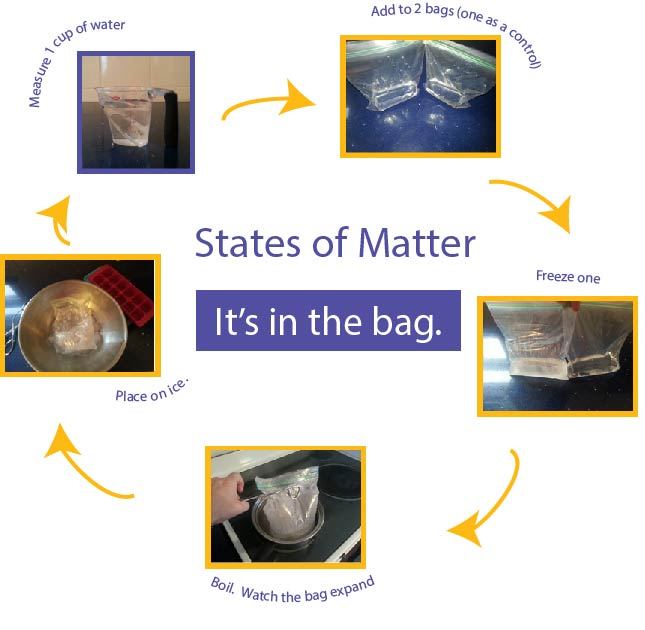
With the first part of this experiment, the student will examine how a liquid changes as it freezes into a solid. Two re-sealable sandwich bags are used as the water changes to the other states and back again.- Measure one cup of water into each bag.
- Remove most of the air from the bags.
- Put one bag in the freezer and leave the other at room temperature. Give several hours for the water to freeze.
- Examine the bags. Note how the ice takes up more of the bag than the water.
Explain that the molecules in the ice got closer together, until they joined in a lattice-shaped form.
Human Demonstration
Illustrate this by holding on to each other with outstretched arms and elbows locked straight out. You are far from each other.The liquid state can be illustrated by pulling your arms in with your elbows bent. You can touch each other's arms with your elbows. You are allowed to move around, but keep touching each other's arms. You are closer together, but moving more. You are also not as tightly connected.
Melting
Melting is one of the easiest transitions of the states of matter to understand.- Let the bag with ice sit out at room temperature.
- Let younger students predict what will happen.
- When the water has melted, notice the water level compared to the mark on the bag.
- Remeasure it to see that it is still one cup.
Use the Evaporation and Condensation MatchCard to illustrate the temperature changes that occur as substances move from the solid to liquid to gaseous state.
Evaporating
Now let's return to our sandwich bag. This can be done with a stove top or microwave. As an alterative, the bag can be placed in a full pot of boiling water.- Be careful to avoid skin burns with this experiment.
- If using the stove top, add water to a pan, bring the water to boil, and add one bag of water to the pan.
- This can be done in the microwave as well.
- Note that as the water evaporates the bag expands from the water vapor.
Alternative Boiling Experiment

- Place the open end of the balloon over the mouth of the bottle, sealing the bottle with the balloon.
- Turn the stove on high, and boil the water.
- Put one half to one cup of water in the bottle.
- Carefully put the bottle into the pan of boiling water.
Human Demonstration
Illustrate this with a group of blind folded people playing group tag in a room. You are not connected, you are moving quickly, you bump into each other, and you take up more space.Condensation
Condensation occurs as a substance in the vapor state (gas) is cooled and returned to a liquid state.- Chill a bowl in the refridgerator or freezer. Or you can put ice cubes in a bowl.
- Put the hot bag expanded with water vapor into the cold bowl.
- Watch the water drops form along the side of the bag that is being cooled.
Alternative Condensing Experiment

Point out the illustrations on the Evaporation and Condensation MatchCard to show the movement of molecules as the states of matter change.
The Hunt
- Brainstorm situations where they have seen substances freeze, melt, evaporate, and condense.
- Make a list.
- Have a six week hunt. See who can point out situations when a substance is moving from one state to another.
- Condensation is the hardest to recognize. Here are a few situations you may wish to point out if the students don't find them:
- condensation on the outside of an ice cold glass on a hot day
- condensation on the inside of a windshield on a cold day
- fog on your glasses when you go into hot air or open the stove
- fog
- dew on the grass
Additional idea: Illustrate or photograph some of these scenarios.
MatchCard Science
How To Use MatchCards
Download the FREE MatchCard Science Instructor's Guide and see how MatchCards can make building their science knowledge base fun.
Light and Energy Unit Study

Download the entire Light and Energy unit study.
12 Science Unit Studies

Chemistry is only one of twelve complete unit studies for kids in 3rd to 8th grade.
Comprehensive objectives, hands-on projects, suggested science fair experiments, and the fun game-like MatchCards keep them interested in learning science. See all twelve MatchCard Science Unit Studies.
About Our Site
Hands-On Learning