Electron Configuration Worksheet
Easy Ways to Learn Chemistry For Kids
The chemistry worksheets are from the MatchCard Science Chemistry Unit Study for grades 3 - 8. In this lesson students learn electron configuration. Chemical symbol and atomic number is reinforced from previous lessons.
Free Download Below

MatchCard Chemistry Worksheets
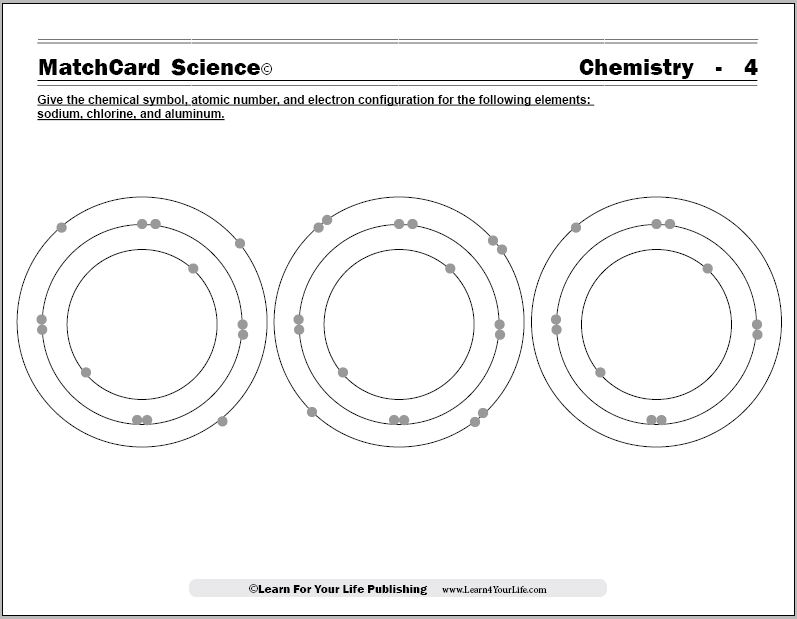
Objective of MatchCard Four: Give the chemical symbol, atomic number, and electron configuration for the following elements:- Sodium
- Chlorine
- Aluminum.
MatchCard Information Pieces identify the name, chemical symbol, and atomic number of three common elements. Information pieces are matched to the model of sodium, chlorine, and aluminum atoms.
How to teach electron configuration with this worksheet:
- Discuss electron configuration (below)
- Build the paper plate models
- Play the electron game with the model
- Use the MatchCard to review the lesson
Download and Use the Chemistry Worksheets for MatchCard #4


This is MatchCard #4 of the Chemistry Unit Study. Find more information on MatchCard Science below.
Chemistry Concepts for Kids
The chemistry worksheets review the concepts that were taught in the previous three lessons:- Parts of the atom and their location in or out of the nucleus
- Atomic number as the number of protons in the nucleus and electrons revolving around the nucleus
- Every element has a distinct atomic number
- Chemical symbol as a universal symbol for each element
The following pages provide more information on:
- Building paper plate models of atoms
- Atomic number and chemical symbol for kids
- Chemistry games to teach atomic number
Electron Pairs on the Chemistry Worksheets
Show the 4th MatchCard to the student. Ask how the diagrams are the same and different. Give them time to study the models and answer.They should recognize that some of the models have more electrons.
Electron Configuration
The chemistry worksheets demonstrate to the student that:- Electrons fill the shells from inner to outer layers.
- The first shell has only two electrons and is filled.
- The second layer has eight electrons and then is filled.
- The second layer will take four single electrons, then begin pairing electrons until four pairs fill the second layer.
- The third layer also is filled with eight electrons. Four single electrons are placed then the next four are paired.
Play the Electron Game
This game will help the students master the concepts of electron configuration.Make three paper plate models of atoms. The nucleus is in the center. Draw three concentric circles - like a bulls eye - to represent three electron shells.
Use paper circles (or pennies) to represent electrons.
In this game, we will not be filling the nucleus with protons and neutrons as was done in earlier games. Explain to the student that it is understood that the protons and neutrons are in the center of the atom.
You will also need one die to play the game.
The first student rolls the die, then takes the number of "electrons" indicated on the die. They have to apply those to one (and only one) of their atomic models. The electron shells must be filled from inside to outside as indicated by the rules of electron configuration above.
After each move, the next player's turn begins.
Each model must be completed with the exact number of electrons needed. For instance, if they need two more electrons to complete sodium, and roll a three, they cannot add to the sodium model. They may add it to another model that still has room for three more electrons.
If another student thinks the rules of electron configuration were broken, they challenge the other student. If the challenge is upheld, that student takes off the pieces applied during that turn. The next player then moves.
The first player to complete all three models with the exact number wins.
MatchCard Science
How To Use MatchCards
Download the FREE MatchCard Science Instructor's Guide and see how MatchCards can make building their science knowledge base fun.
Chemistry Unit Study

Explore the building blocks of matter with the chemistry unit study.
12 Science Unit Studies

Chemistry is only one of twelve complete unit studies for kids in 3rd to 8th grade.
Comprehensive objectives, hands-on projects, suggested science fair experiments, and the fun game-like MatchCards keep them interested in learning science. See all twelve MatchCard Science Unit Studies.
About Our Site
Hands-On Learning