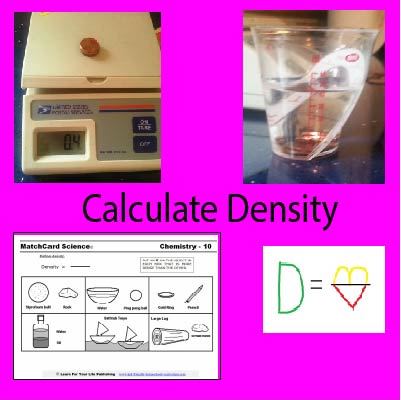
Density Formula
Use MatchCard Science density worksheet to teach the density formula.
Free Download Below

What Is Covered?
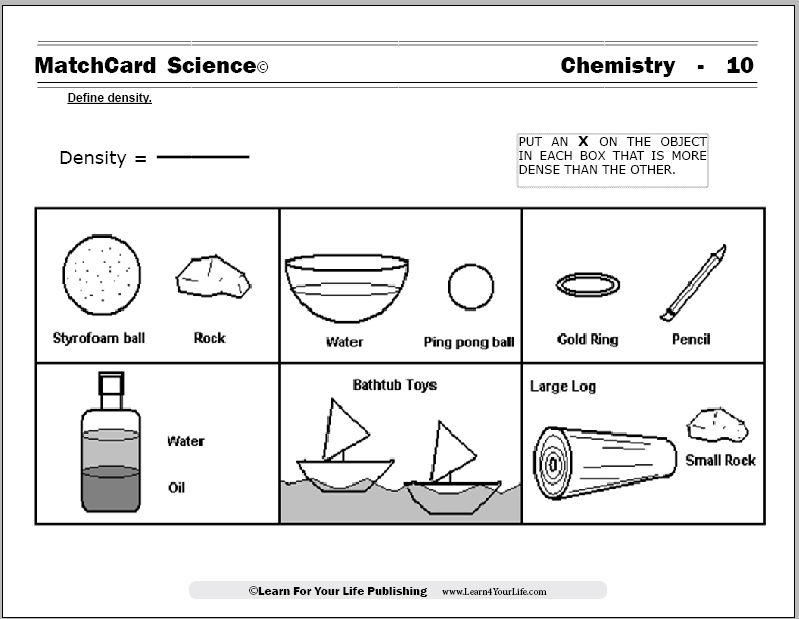
Objective: Define Density.MatchCard: Download below.
MatchCard Information Pieces are used to create the density formula (mass divided by volume) and identify which objects have greater density.
Projects: Given a set of irregular objects, students arrange them by their estimates of most to least dense. Calculate the density of the objects using a scale and water displacement. Learn a mnemonic for the density formula.
Here's Your Copy of the Density Formula MatchCard


This is MatchCard #10 of the Chemistry Unit Study. Find more information on MatchCard Science below.
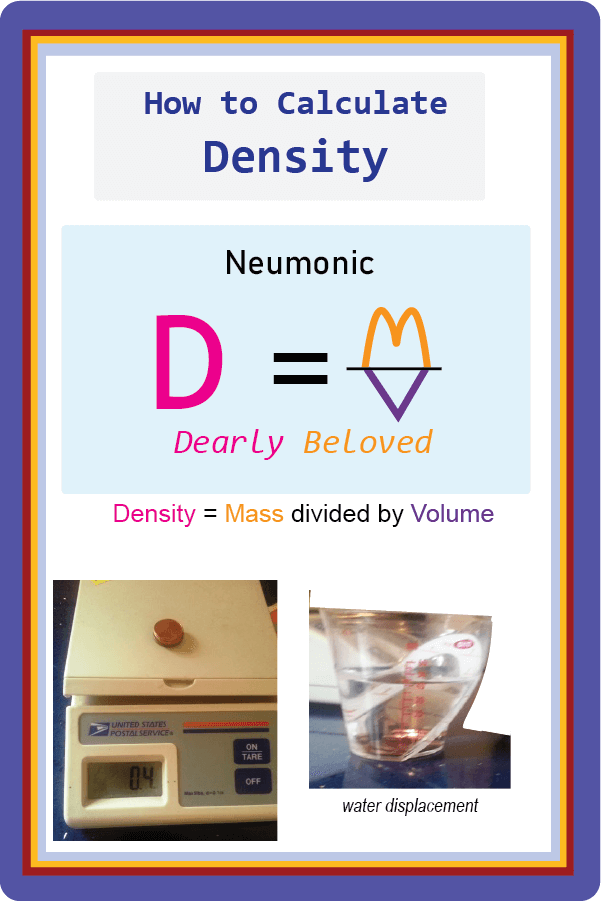
Let's Learn the Density Formula
Density equals Mass divided by Volume
Mass
___________
Volume
Introduce Density
Opening Activity: With a student blindfolded, put a small dense object (like a metal coin) on one plate or cakepan they are holding in one hand, and a large lightweight object (like a basketball or plastic dog toy) on the identical plate or pan in the other hand. Ask them which has greater mass.The answer should be the larger item.
When they open their eyes, ask if metal or plastic has greater mass. Which would float in water? How is it that the plastic was heavier?
They will likely try to explain that the light-weight object had more mass because there was more of it.
Then ask: "How can we compare the mass of these two objects of different sizes?"
The Density Formula
Explain that chemists use the density formula to compare the mass of objects. The formula is listed above: mass divided by volume.So what two things do we need to determine density?
- Mass
- Volume
Hands On Learning With the Density Formula
Students benefit from practice using density. First, give them a set of relatively small objects with various densities. These objects could include:
- Different coins
- Several small or medium rocks
- Marble, lego, small toys
- Key
- Paper clip
Basically, any items that will fit in a cup and do not dissolve in water will work. Try to get five to eight different items to work with.
Estimate density
Let them make a list of the items: from most to least dense. Have them guess while handling the objects.
Write it down, so they can compare it later.
Determine mass

If you do not have an accurate enough scale, you may need to measure a number of items. For instance, when measuring the density of pennies, it helps to get the mass and volume of four or five pennies rather than just one, unless you have a scientific scale with precision for tenths of grams.
Determine Volume

You may be able to measure by dropping the object into a beaker or cylinder and determining how much the volume in the container increases by milileters.
The best way is to use water displacement. It would be great if you happened to have a water displacement measurer. But in the very likely case you do not have one, here is how to make one:
- Puncture a hole in a disposable or Styrofoam cup.
- Place the cup inside a measuring cup that has metric measurements (usually milliliters).
- Fill the cup to the level of the hole.
- Drop the object into the cup. It will displace the volume of the object so that the same volume of water spills out of the hole and into the measuring cup.
- Measure the volume of water in the cup.
Liquid volume of milliliters (ml) can be converted to solid volume of cubic centimeters (cc).
Determine Density
Now use the density formula of mass divided by volume to determine the density of each of your objects. List them from most to least dense.Now compare the list of actual densities to their list of estimates before the activity. How close were they?
Trick for Remembering the Density Formula
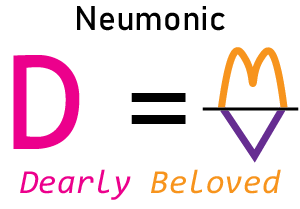 It’s an old mnemonic, but it works! Students can remember the formula by thinking of the phrase “dearly beloved”. (Some people use the phrase “dumb love” or even “dense love” but I think “dearly beloved” is a little nicer, even if a bit archaic sounding.).
It’s an old mnemonic, but it works! Students can remember the formula by thinking of the phrase “dearly beloved”. (Some people use the phrase “dumb love” or even “dense love” but I think “dearly beloved” is a little nicer, even if a bit archaic sounding.).First, write down the density formula using only the first letter of the three words. It would be D = M/V. (Don't make the division sign at a slant but straight across, parallel to the bottom of the paper.)
The "D" stands for "dearly" or "dear." Then draw the M and V to look like a heart.
The "M" forms the top of the heart. Draw it so it looks like the famous golden arches of a fast food restaurant.
Then draw the "V" as the bottom of the heart.
Now you have the density formula in a picture they will never forget!
MatchCard Science
How To Use MatchCards
Download the FREE MatchCard Science Instructor's Guide and see how MatchCards can make building their science knowledge base fun.
Chemistry Unit Study

Explore the building blocks of matter with the chemistry unit study.
12 Science Unit Studies

Chemistry is only one of twelve complete unit studies for kids in 3rd to 8th grade.
Comprehensive objectives, hands-on projects, suggested science fair experiments, and the fun game-like MatchCards keep them interested in learning science. See all twelve MatchCard Science Unit Studies.
About Our Site
Hands-On Learning