Conservation of Mass
The Conservation of Mass worksheet is from MatchCard Science Chemistry Unit Study for grades 3 - 8. Kids discover how matter is changed in a chemical reaction without being destroyed.
Free Download Below


MatchCard Science Conservation of Mass
Objective: Explain the law of conservation of mass.MatchCard: Download below.
Students give examples of conservation of mass and answer true and false questions.
Projects: Sizzle and Burn. Burn a log or sheet of paper. Dissolve an seltzer tablet and weigh the results.
Download and Use the Conservation of Mass MatchCard


This is MatchCard #11 of the Chemistry Unit Study. Find more information on MatchCard Science below.
Introduce the Conservation of Mass
Fire


Ask what happens to wood when it burns. It looks like the wood is destroyed.
When a piece of wood is burnt in a fireplace, only a small amount of ashes are left. It appears that the fire destroyed the wood and that most of the substance in the wood is completely gone.
As an alternative, it is possible to burn a piece of paper in an outdoor grill or other appropriate container. Obviously, discuss fire safety and appropriate use of matches with kids as needed. We are going to do a little experiment to show that matter is never completely destroyed - at least in a scientific sense.
Sizzling Tablets
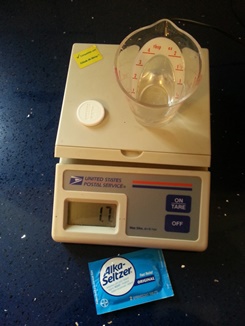
- a small scientific, kitchen, or postal scale
- Disposable bathroom cup or other small, light weight container
- One quarter cup of water
- One Alka-seltzer tablet
- One small ziploc bag
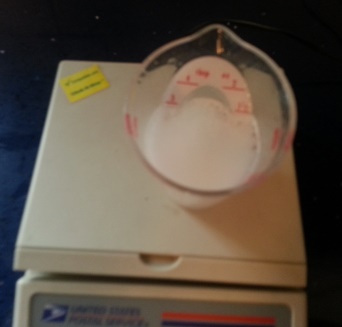
Now, drop the tablet in the water. Watch the chemical reaction.
When the sizzling is finished, weigh the cup again.
Oops, it weighs less than the combined weight before they were added. It LOOKS as if some matter disappeared.
Now, let's do it again and see what happened to that missing weight. Add one quarter cup of water into a small ziploc bag. Seal the bag and weigh it.
Now drop the tablets into the bag and seal it QUICKLY. Enjoy watching the experiment again.
When you weigh the ziploc bag after the reaction is done you will find it is the same as the weight of all the substances before the experiment. Ask the student to explain the difference. They should be able to discern that the gas that escaped in the chemical experiment was still in the bag but not in the cup.
It's a neat little example of the conservation of mass. The chemical matter was changed, but it still existed in a different form.
Learn About the Conservation of Mass
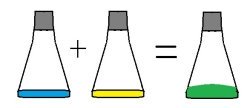 Another example is burning of coal. Use the information pieces from Chemistry Matchcard 11 (download below) to demonstrate this principle.
Another example is burning of coal. Use the information pieces from Chemistry Matchcard 11 (download below) to demonstrate this principle.Coal is made up of carbon. Put the "C" for carbon on the table.
When coal is burned in a coal burning stove, it looks like it is destroyed just as it looks like wood is destroyed in a fireplace. But the chemical matter does not disappear. A substance can only burn in the presence of oxygen. Put "O2" chemical symbol on the table near the symbol for carbon.
When coal is burned, the carbon combines with the oxygen to produce carbon dioxide. Place the CO2 symbol on the table. Carbon dioxide is a gas and we can't see it. But neither the carbon nor the oxygen were destroyed. The atoms reformed into different molecules, but the atoms still existed.
The conservation of mass tells us that the number of atoms after a chemical reaction will always be the same as the number before the reaction. Substances change, but they are not destroyed.
Carbon Monoxide
Teacher’s Note: Sometimes the burning of coal results in carbon moxoide where 2 carbon atoms react with 1 oxygen molecule to produce 2 carbon monoxide molecules. The chemical formula is (2)C + 02 = (2)CO. This formula is a little more complex.What's In A Name?
“Conserve” means to keep or save. “Conservation” is the noun form of the word “conserve.” Can you explain the phrase “Conservation of Matter?”MatchCard Science
How To Use MatchCards
Download the FREE MatchCard Science Instructor's Guide and see how MatchCards can make building their science knowledge base fun.
Chemistry Unit Study

Explore the building blocks of matter with the chemistry unit study.
12 Science Unit Studies

Chemistry is only one of twelve complete unit studies for kids in 3rd to 8th grade.
Comprehensive objectives, hands-on projects, suggested science fair experiments, and the fun game-like MatchCards keep them interested in learning science. See all twelve MatchCard Science Unit Studies.
About Our Site
Hands-On Learning