Conservation of Energy
Analyze 9 examples of Conservation of Energy
Free Download Below
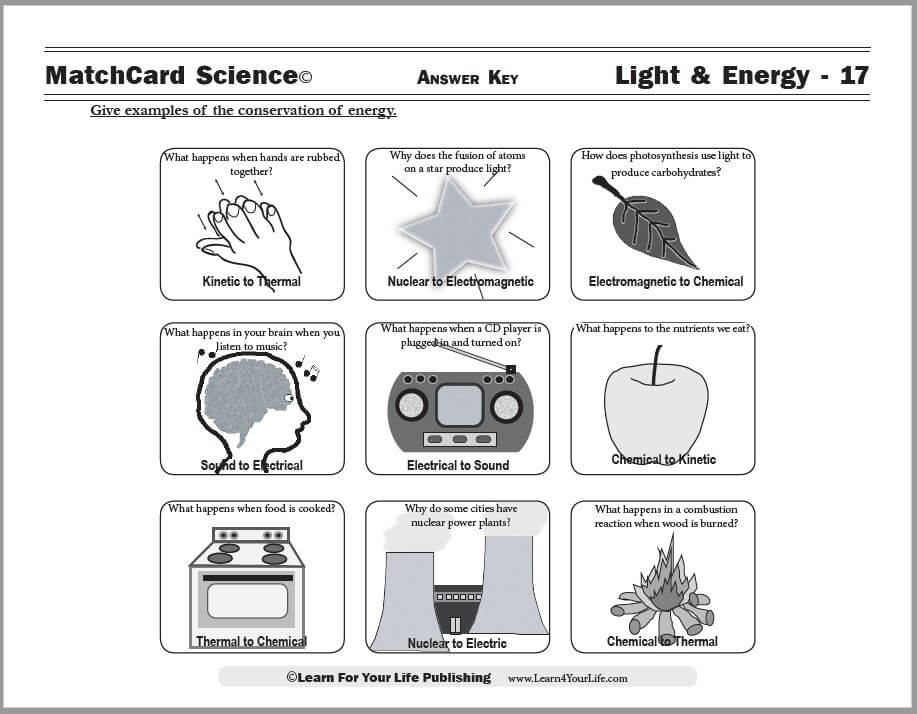

MatchCard Science Conservatioh of Energy Worksheett
Objective: Give examples of of the conservation of energy.MatchCard: Downloads below.
Print the Conservation Of Energy Worksheet


Click image to go to download.
This is MatchCard #17 of the Light & Energy Unit Study. Find more information on MatchCard Science below.
The Law of Conservation of Energy
In a closed system, energy cannot be created or destroyed. Energy can be changed from one form to another.Energy Transformation
One type of energy can be converted to another type of energy. In this lesson, we will learn about several types of energy transformation. But there are many more that you can find all around you.Energy Sources
You cannot “create” energy. But you can change energy from another form. A windmill is an example of an energy source that takes kinetic energy and turns it into electrical energy. A physicist studies energy in order to calculate the amount of energy in the new form.Examples of Conservation ot Energy on the MatchCard
Kinetic to Thermal

The kinetic energy of your hands was changed to thermal energy.
Nuclear to Electromagnetic
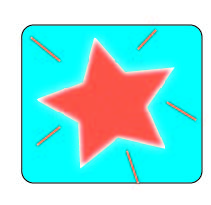
Wow! Imagine being a physicist and trying to calculate that amount of energy. Obviously, they have to do it from a distance.
Electromagnetic to Chemical

Sound to Electrical
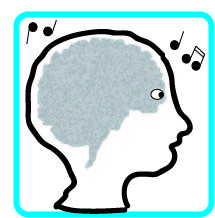
Electrical to Sound
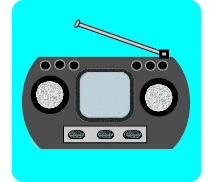
Chemical to Kinetic
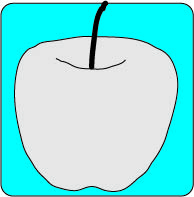
Electrical to Thermal

Nuclear to Electrical
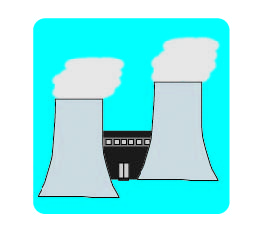
Chemical to Thermal

Teacher's Note: Nuclear Energy
With most of the seven different types of energy, we have provided at least one example of it being the source of energy and the product of energy transformation. The exception is nuclear energy. While nuclear energy is the source for other types of energy, it is not within the range of normal conditions on Earth that nuclear energy is created. Of course, on the sun you can say that thermal energy is converted to nuclear energy which is converted to electromagnetic energy.This demonstrates how the sun is the source of all energy on our planet. Solar energy panels directly convert to the sun's rays to usable energy. But most other forms go through other conversions.
Supplemental Activities
Name that Energy Transfer
How many other forms of energy transfer you can think of? Make a list. Perhaps on a long car trip you can identify different types of energy and energy transfer that you see out the window.Energy Dot to Dot Game
Did you notice that in some examples a type of energy that is produced becomes the source of another energy transformation. Make a game whereby one person lists an energy transformation, and the next person has to list another transformation starting with energy produced in the previous turn.Use MatchCard #16: Types of Energy as a visual reminder of the different types of energy you can use.
- One person names a type of energy: for instance "thermal."
- The next person may say, "Thermal energy is converted to kinetic energy in a steam engine."
- The next person may say, "Kinetic energy is converted to electrical energy by a windmill."
Super Challenge: They have to start at the beginning and recite all the previous examples in the correct order.
Energy Curse
A fun book you might get at the library is Math Curse by Jon Scieszka. It is the story of a student who starts to go bananas as she sees all the world as math problems. At the end she escapes her dilemna of seeing all the world mathematically, and is presented with the dilemna of seeing all the world as a science problem. Perhaps you can write a sequel, by viewing the world as a series of energy problems.Discussion: Things to Know & Note
1st Law of Thermodynamics
This is a famous formula based on the Law of Conservation of Energy. It states that the amount of change of energy in a system is equal to the energy supplied minus the amount of work done. It could be written this way:Energy supplied to a system - Work done = Change in amount of energy
Perpetual Motion Machine
According to the Law of Conservation of Energy AND the 1st Law of Thermodynamics, there can never be a perpetual motion machine (a machine that runs without someone supplying energy.) Many people have tried to invent a perpetual motion machine but none have been successful. How would you like it if you could be the first person to invent such an impossibility? If you could make on contribution to the field of energy and physics, what would you l ike it to be?MatchCard Science
How To Use MatchCards
Download the FREE MatchCard Science Instructor's Guide and see how MatchCards can make building their science knowledge base fun.
Light and Energy Unit Study

Download the entire Light and Energy unit study.
12 Science Unit Studies

Chemistry is only one of twelve complete unit studies for kids in 3rd to 8th grade.
Comprehensive objectives, hands-on projects, suggested science fair experiments, and the fun game-like MatchCards keep them interested in learning science. See all twelve MatchCard Science Unit Studies.
About Our Site
Hands-On Learning