Chemistry for Kids
Make a model of hydrogen and helium atoms.
These chemistry for kids worksheets are from the MatchCard Science Chemistry Unit Study for grades 3 - 8. In this worksheet, students will learn the name, chemical symbol, and number of protons in hydrogen and helium atoms.
Free Download Below
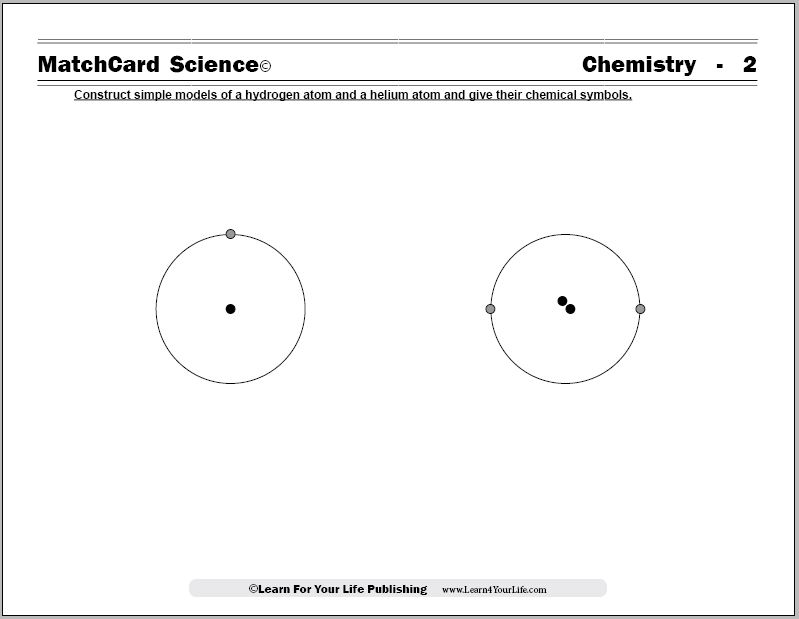

Learn the Parts of Hydrogen and Helium Atoms
Objective: Construct a simple model of a hydrogen atom and a helium atom and give their chemical symbols.MatchCard: Download below.
A simple diagram of a hydrogen atom and helium atom is on the student copy of the MatchCard. Information pieces will be matched which identify the name, chemical symbol, and differentiate the number of protons in the nucleus.
Projects: Continue building paper plate models of atoms. Have a guessing game on the number of elements. Introduce hydrogen and helium.
Download the Hydrogen and Helium MatchCard


This is MatchCard #2 of the Chemistry Unit Study. Find more information on MatchCard Science below.
The Chemistry for Kids MatchCard
In the first MatchCard lesson, students used paper plates to build a model of an atom. In this lesson, they will use their paper plate models to differentiate hydrogen and helium atoms.Review
Review the following information from the first Chemistry Matchcard:- Protons are in the nucleus in the center of the atom
- Neutrons are in the center with the protons
- Electrons circulate around the outside of the atom.
- There are the same number of protons and electrons in an atom.
Let's Guess
Explain to your student(s) that different elements are chemicals with different atoms. Ask this simple question:"How many different elements do you think there are in the world?"
Give them time to make some guesses.Answer: At this time there are only 117 known elements that chemists have found. Of those 117, only 86 are common elements.
This would be a good time to show the periodic table and explain that each box represents one and only one element.
- Just as 26 different letters make up millions of different words of different sizes, these 86 elements make up all the many different substances you see every day.
- Each different element has a different size atom.
- All atoms of the same element have the same number of electrons and protons.
- It is the number of protons and electrons in an atom that determine what chemical the substance is.
- Neutrons do not influence the atomic number, so we will ignore them for several lessons. But don't forget they are still there in the nucleus, hanging out with the protons.
- Because the names of atoms can get long (and difficult to spell) there are chemical symbols for each element.
Make Your Atomic Models
In this chemistry for kids lesson, students will make paper plate models of the two simplest atoms: hydrogen atoms and helium atoms.Use the same circles with "P", "E", and "N" for this activity. You will also need two paper plates. (Circles traced from dinner plates on plain paper also work.)
Hydrogen Atom
Hydrogen is the simplest atom. It has only one electron and one proton.Have you ever heard of hydrogen? Hydrants, and hydration refer to water. Water has hydrogen in it.
The chemical symbol for hydrogen is "H".
Helium Atom
The helium atom has two electrons and two protons. It's chemical symbol is "He".Ever heard of helium? Yep, this is the element that fills helium balloons.
Hydrogen and helium are both gases.
Helium Project
Helium makes balloons fly, right? Helium is very light weight (after all, it only has two protons) and is lighter than air. So it rises upward. If the helium escaped from it’s container, it would head to outer space pretty quickly. However, when we put it in a balloon, the weight of the balloon weighs it down. It slowly escapes, and the helium happily makes its way out of Earth’s atmosphere, while the child has to sadly contend with a floppy balloon.Project: Does helium escape from a latex balloon or mylar balloon faster?
Next Lesson in Chemistry
Our next lesson we'll learn atomic number and chemical symbols while playing a game and continuing to build our models.MatchCard Science
How To Use MatchCards
Download the FREE MatchCard Science Instructor's Guide and see how MatchCards can make building their science knowledge base fun.
Chemistry Unit Study

Explore the building blocks of matter with the chemistry unit study.
12 Science Unit Studies

Chemistry is only one of twelve complete unit studies for kids in 3rd to 8th grade.
Comprehensive objectives, hands-on projects, suggested science fair experiments, and the fun game-like MatchCards keep them interested in learning science. See all twelve MatchCard Science Unit Studies.
About Our Site
Hands-On Learning