Solid Liquid Gas Worksheet
States of Matter MatchCard
The Solid Liquid Gas Worksheet from MatchCard Science Chemistry Unit Study explores the states of matter.
Free Download Below
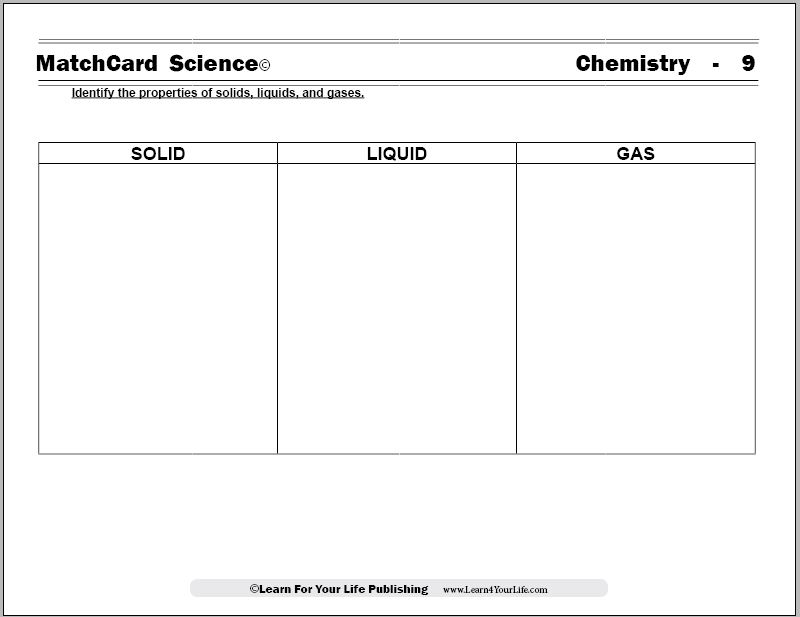

MatchCard Science Solid Liquid Gas Worksheet
Objective: Identify the properties of the solid, liquid, and gaseous states.MatchCard: Download below.
MatchCard Information Pieces identify the shapes and molecular behavior of substances in the solid state, the liquid state, and the gaseous state. Students match the descriptions with the correct state of matter.
Projects: Measure the temperature that water changes to ice and when it evaporates. Add other chemicals to see if it affects the temperature where the state of matter changes.
Print the States of Matter Worksheet


This is MatchCard #9 of the Chemistry Unit Study. Find more information on MatchCard Science below.
Let's Get Started
Have ice cubes and a glass of water on hand. Ask the student to tell how they are alike and how they are different.Answer: They are alike because they are made out of H2O. They are different because the ice is colder and frozen.
You may wish to review the Freezing, Melting, Evaporation, Condensation MatchCard to identify how temperature changes the states of matter.
Measure the Temperature Changes
Use a scientific thermometer to measure the temperature of the water when no ice is left in the glass. This will require checking the temperature at least every five minutes.This is the melting temperature of ice.
Now, put the water in a pan and turn the stove on it's lowest setting. Again, measure the temperature when the water starts to boil and evaporate.
This is the boiling/evaporating temperature of water.
Other Substances
Every substance has a melting/freezing temperature and a evaporating/condensing temperature.With some substances, the melting temperature is so high we cannot turn it into a liquid or gas on this planet.
Likewise, some gases have an evaporating/condensing temperature that is so low, there is no chance of it turning into a liquid on Earth.
Let's Experiment
If you add common chemicals to water, does it change the boiling or freezing point. Design an experiment to find out.Here's some chemicals you might try to add:
- Salt
- Vinegar (an acid)
- Baking Soda (a base)
- Iodine
Physical Properties of Matter
All liquids have certain things in common in regard to their shape and behavior of molecules. The same with solids and gases.Solids
The shape of a solid does not change unless an outside force is applied to it. Solids maintain there own shape.Molecules in a solid are held tightly together and vibrate slowly.
Liquid
Substances in a liquid state take the shape of the container. A liquid has no shape of its own.Molecules in a liquid are held loosely together and vibrate rapidly.
Gas
A gas has no shape. Particles move in all directions and expand outside of the walls of a container.The molecules in a gas are not connected and are in constant motion.
MatchCard Science
How To Use MatchCards
Download the FREE MatchCard Science Instructor's Guide and see how MatchCards can make building their science knowledge base fun.
Chemistry Unit Study

Explore the building blocks of matter with the chemistry unit study.
12 Science Unit Studies

Chemistry is only one of twelve complete unit studies for kids in 3rd to 8th grade.
Comprehensive objectives, hands-on projects, suggested science fair experiments, and the fun game-like MatchCards keep them interested in learning science. See all twelve MatchCard Science Unit Studies.
About Our Site
Hands-On Learning