Acids and Bases
Acid Base Worksheet From MatchCard Science
Use the acids and bases worksheets and homemade litmus paper to teach the properties of acidic and alkali substances.
Free Download Below
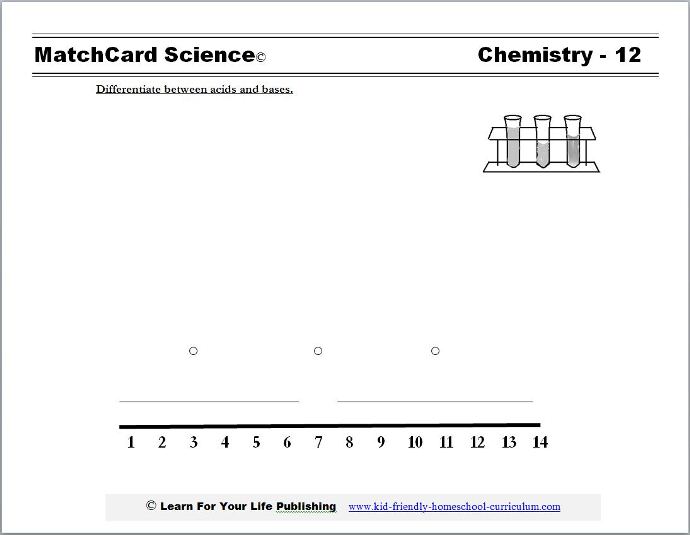

MatchCard Science Acid Base Worksheet
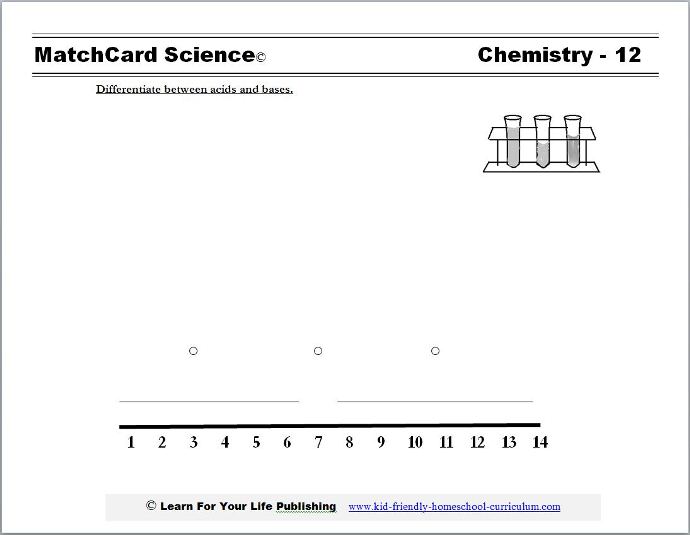
Objective: Differentiate between acids and bases.MatchCard: Download below.
Identify acids, bases, and neutral substances.
Projects: Make homemade litmus paper. Test different substances. Combine acids and bases and watch the chemical reaction.
Download and Use the Acid Base MatchCard


This is MatchCard #12 of the Chemistry Unit Study. Find more information on MatchCard Science below.
Acids and Bases Experiments
Experiments with acids and bases are common chemistry projects, and students become acquainted with the chemical properties of acids and bases. Our first experiment will help students differentiate between acids and base substances. The second experiment will combine acids and bases to watch them react.
Make Your Own Litmus Paper

1. Get A Red Cabbage

2. Boil the Cabbage Leaves

3. Soak Index Cards in the Cabbage Solution

4. Cut Index Cards Into Strips

Gather Substances to Test

Common Acids
Here are some common household acis you can collect for the acid base experiments:- vinegar
- Lemon juice (any citrus juice)
- Grape juice or wine
- Boric Acid
- Sour Milk
- Cream of Tartar (dissolve in small amount of water)
- Aspirin (dissolve in small amount of water)
- Cider
- Carbonated beverage, soda water, or seltzer
- Acid Rain
- Stomach acid (ok, maybe you don't want to test this - but add it to your discussion later.)
Common Bases
- Baking Soda
- Toilet bowl cleaner
- Liquid detergent
- Ammonia
- Egg Whites
- Toothpaste
- Milk of Magnesia, Tums, or any other anti-acids (duh!)
Common Neutral Substances
- Water (including rain without a lot of pollution)
- Blood
- Salt Water
Testing Substances with Your Acid Base Indicator

Acids will turn the litmus paper red. Bases turn the paper green. Neutral substances make the paper darker but do not turn it a different color.
If it is determined that it ia substance is an acid, move to one side of the table marked "acid." Move the bases to the other side which should be marked "base." Anything that does not have a color change should be placed in the middle which is marked "neutral."
Which is stronger?
Give them a copy of the Acid Base MatchCard. Based on the properties of acids and bases (below) they can arrange the items they tested as to which they believe are the most acidic or the most basic.
Write down the order that they put the substances in. Save this paper.
Testing with Commercial Litmus Paper
Using scientific litmus paper does more than just determine if a substance is an acid or base. It will give you the pH.Neutral substances have a pH of 7.
Acid substances have a pH of 6 or less. The closer to 1, the stronger the acid.
Base substances have a pH of greater than 8. The larger the number, the stronger the base.
If you do not have litmus paper, you may look up the pH of the different substances with a chemistry textbook or internet search. The MatchCard Learning Activities includes the ph of 19 substances commonly available and tested.
Now compare their guesses with the actual pH. Were they close?
Let's React
Acids and bases react when they are combined. One of the most common experiments is to combine vinegar and baking soda.Let students combine different acidic substanceds and basic substances. The stronger the acid or base, the more dramatic the reaction.
What will happen if you combine an acid with an acid? A base with a base? Let them try. (Boring. No reaction.)
Properties of Acids and Bases
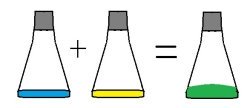
Properties of Acids
Acids are chemical substances that have additional hydrogen (H+) ions.Other properties of acids:
- They break apart in water to form hydrogen ions (the hydrogen breaks off from the rest of the molecules).
- They have a positive charge.
- They are caustic and corrosive.
- They are sour - though no one should taste them unless it is known to be a safe substance (orange juice, for instance.)
- They burn on the skin.
- They react with bases.
- They have a pH less than 7.
- They turn litmus paper red (Remember: Red Hot Acids).
Properties of Bases
Bases are the opposite of acids. They donate rather than accept electrons.Other properties of acids:
- They release (OH-) ions in water.
- They have a negative charge.
- They feel slippery.
- They tend to have a bitter taste, but don't taste them unless you know they are safe.
- They react with acids.
- They have a pH greater than 7.
- They are referred to as "alkaline" substances.
- They turn litmus paper blue. (Remember: Blue Base)
About pH
pH always seems backwards. I mean, acids with their extra H+ ions should have a high number and the bases with fewer H+ ions should have a low number.Here’s why:
pH=-Log10(aH+)
That explained it, right? Here’s a simpler explanation:- Quantity A. 1.5 x 10-2 = 0.015
- Quantity B. 1.5 x 10-10 =0.00000000015
A has a whole lot more H+ atoms than B. Since pH is a negative logarithm, a pH of 2 occurs with more hydrogen ions than a pH of 10. So the acidic solution has a lower pH but more hydrogen ions.
The complete mathematical equation is a bit more complex, but this explanation is sufficient to prevent most of us from losing sleep wondering why more hydrogen ions have a lower pH.
Don’t you feel better now?
MatchCard Science
How To Use MatchCards
Download the FREE MatchCard Science Instructor's Guide and see how MatchCards can make building their science knowledge base fun.
Chemistry Unit Study

Explore the building blocks of matter with the chemistry unit study.
12 Science Unit Studies

Chemistry is only one of twelve complete unit studies for kids in 3rd to 8th grade.
Comprehensive objectives, hands-on projects, suggested science fair experiments, and the fun game-like MatchCards keep them interested in learning science. See all twelve MatchCard Science Unit Studies.
About Our Site
Hands-On Learning